Magnetically Guided Atherectomy
a magnetic guide and atherectomy technology, applied in the field of removing occlusive material from the lumen of the body, can solve the problems of vascular occlusion and the inability to guide the balloon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
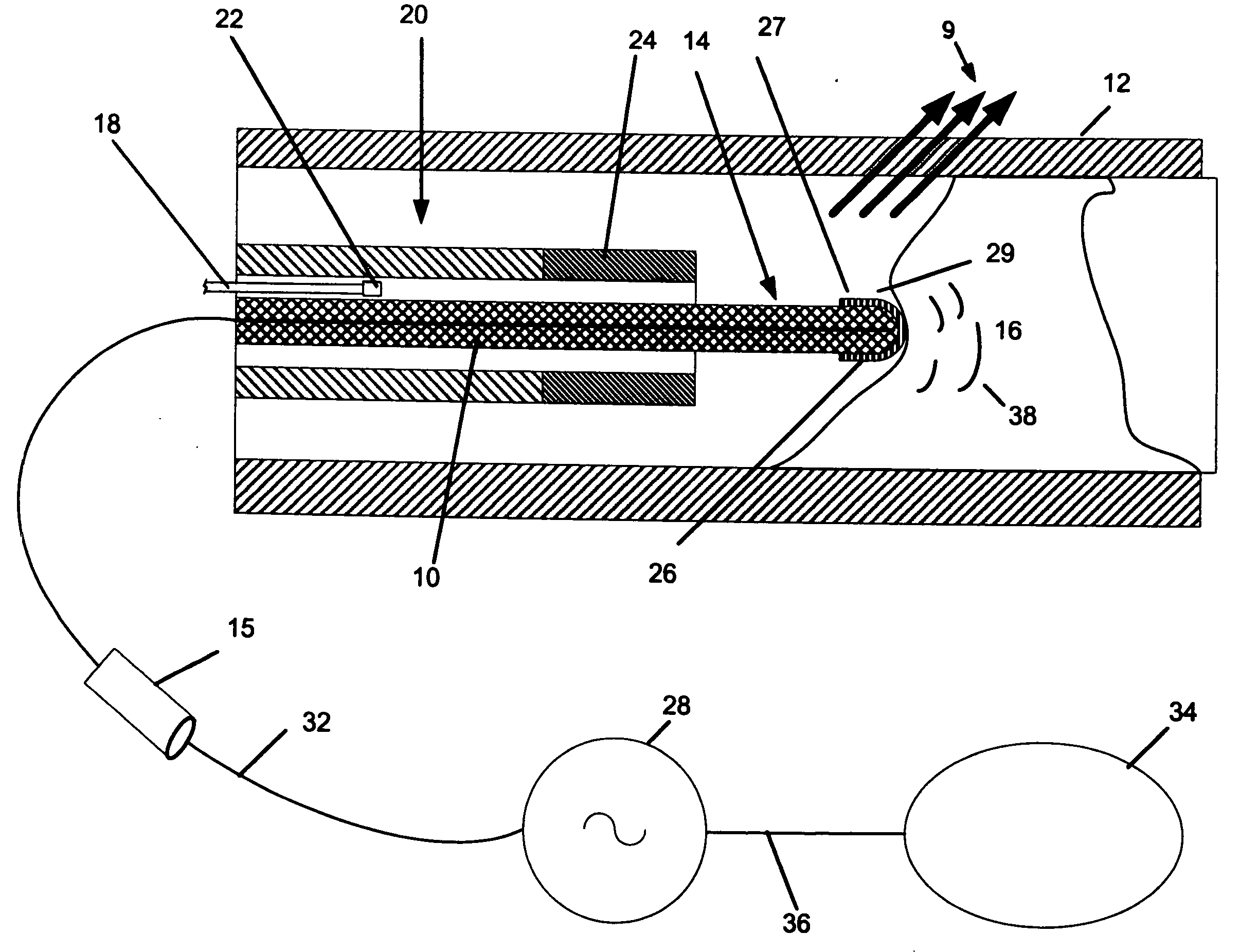
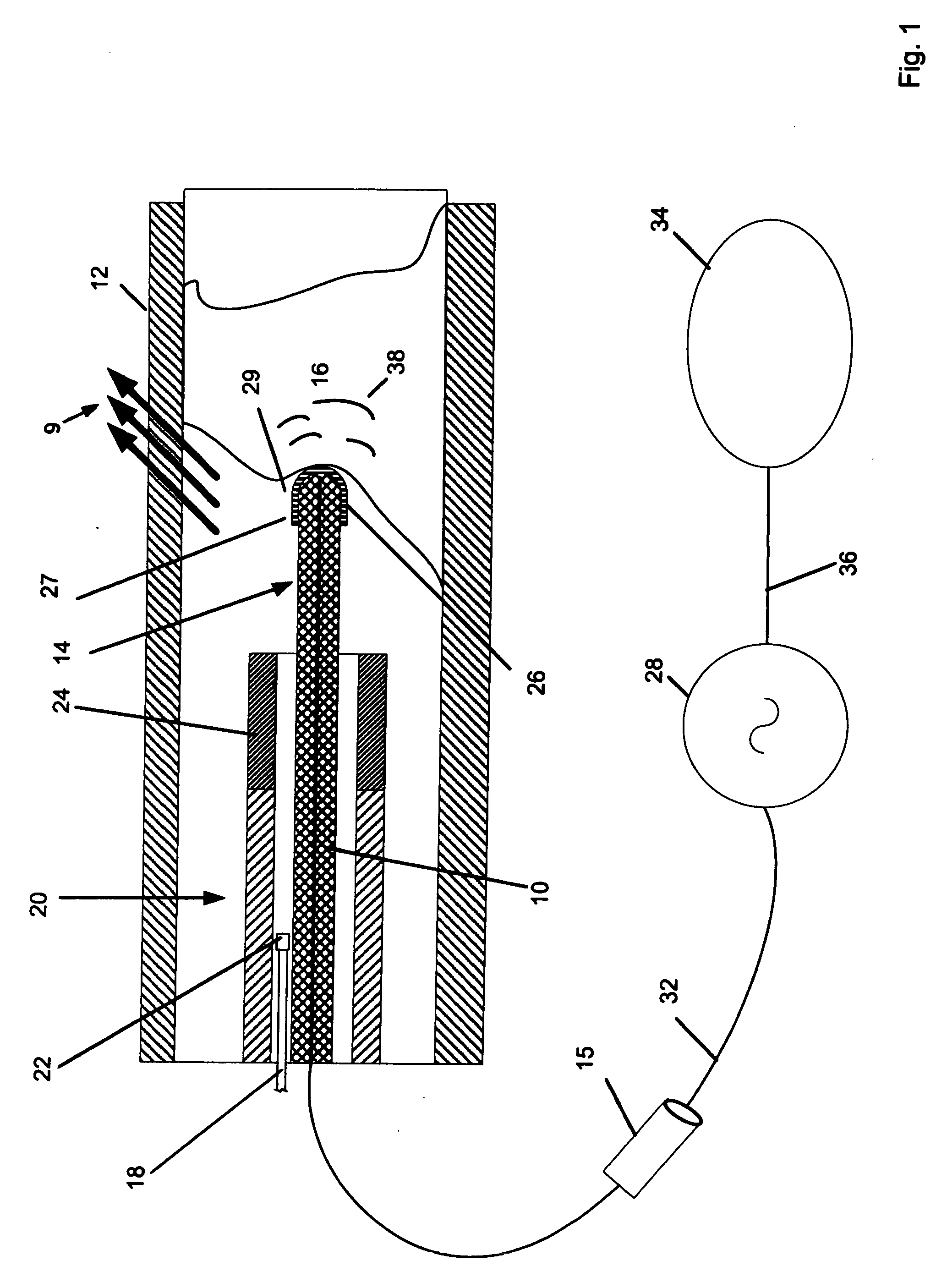
[0029]FIG. 1 shows a thermal catheter 10 in a vessel 12. The distal tip section 14 of the device is shown in a vessel while the proximal section 15 is illustrated as a fragment located outside of the vessel 12. In general, the construction of the proximal end of the device and configuration and power couplings are within the ordinary skill of this art and are illustrated schematically in FIG. 1. For clarity the detailed disclosure is directed to the distal tip structures. However it should be recognized that the devices are intended for use in coronary vessels, the overall length of devices in accordance with this invention are 30 or more inches long and typical are between 2 and 12 French in diameter. It should be understood that coronary use is merely illustrative and other vessels and body lumens may be addressed therapeutically using the invention. The proximal end will carry suitable hubs and connections for the wires and lumens discussed in connection with the distal tip.
[003...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com