Medical implant or medical implant part comprising porous UHMWPE and process for producing the same
a technology of medical implants and porous uhmwpe, which is applied in the field of medical implants and medical implant parts, can solve the problems of affecting the implantation process, and affecting the implantation process, and putting the risk of orthopaedic implants comprising ultrahigh molecular weight polyethylene components that must be anchored to the host bone at risk
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
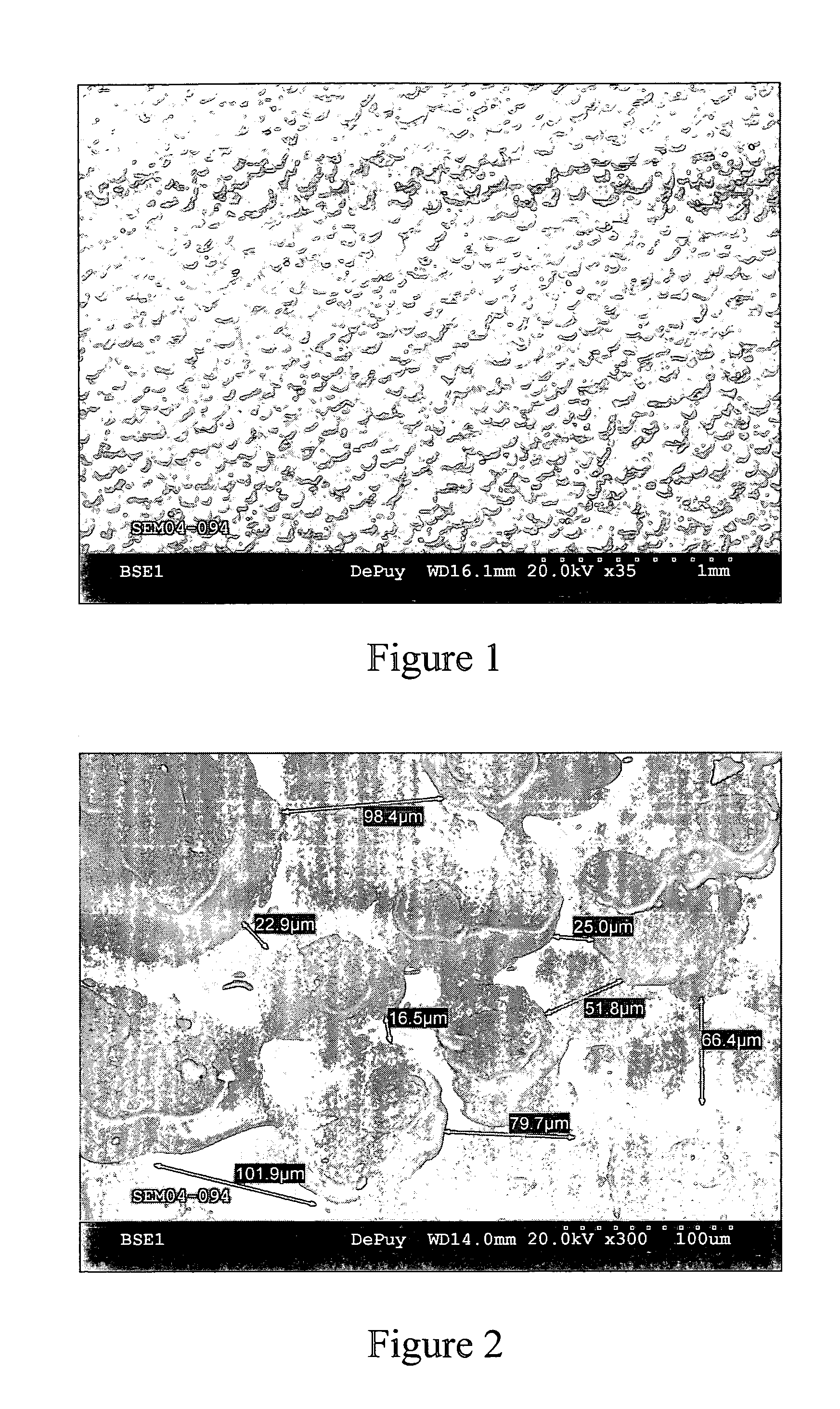
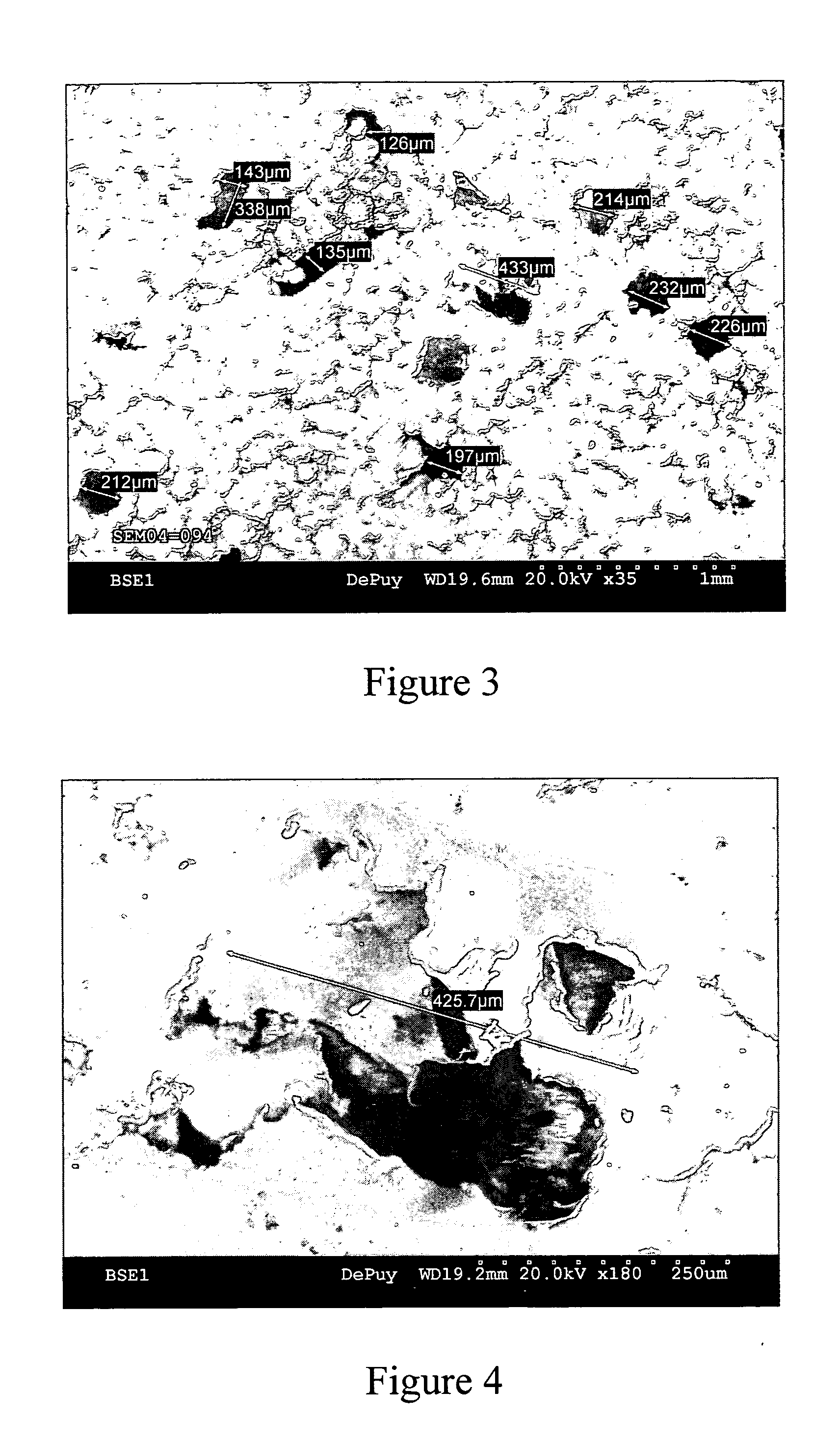
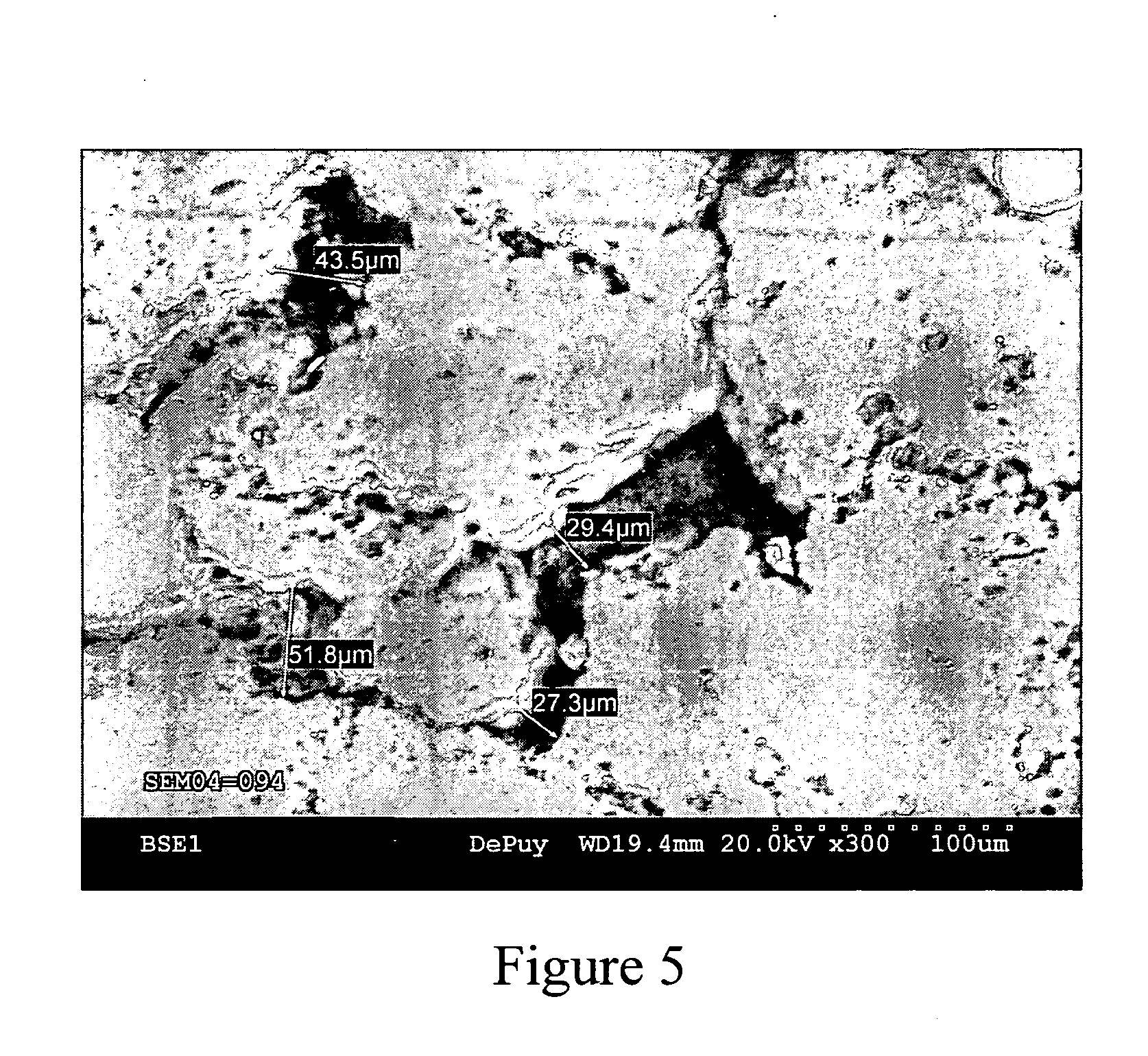
[0044] This example further illustrates the invention but, of course, should not be construed as in any way limiting its scope. This example demonstrates a process for producing a medical implant or medical implant part according to the invention, as well as the properties of the medical implant or medical implant part of the invention. Three samples of porous ultrahigh molecular weight polyethylene (Samples 1-3) were prepared from GUR 1020 powdered ultrahigh molecular weight polyethylene using three different processes.
[0045] Sample 1 (comparative) was prepared by a sintering process in which 14 grams of GUR 1020 powdered ultrahigh molecular weight polyethylene was placed into a cylindrical mold (approximately 10 cm in diameter) and heated in a high-vacuum oven to a temperature of approximately 200° C. under a load of approximately 5.6 kg for 180 minutes. The resulting sample was measured and weighed to determine its porosity, which was determined to be approximately 23%. The surf...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com