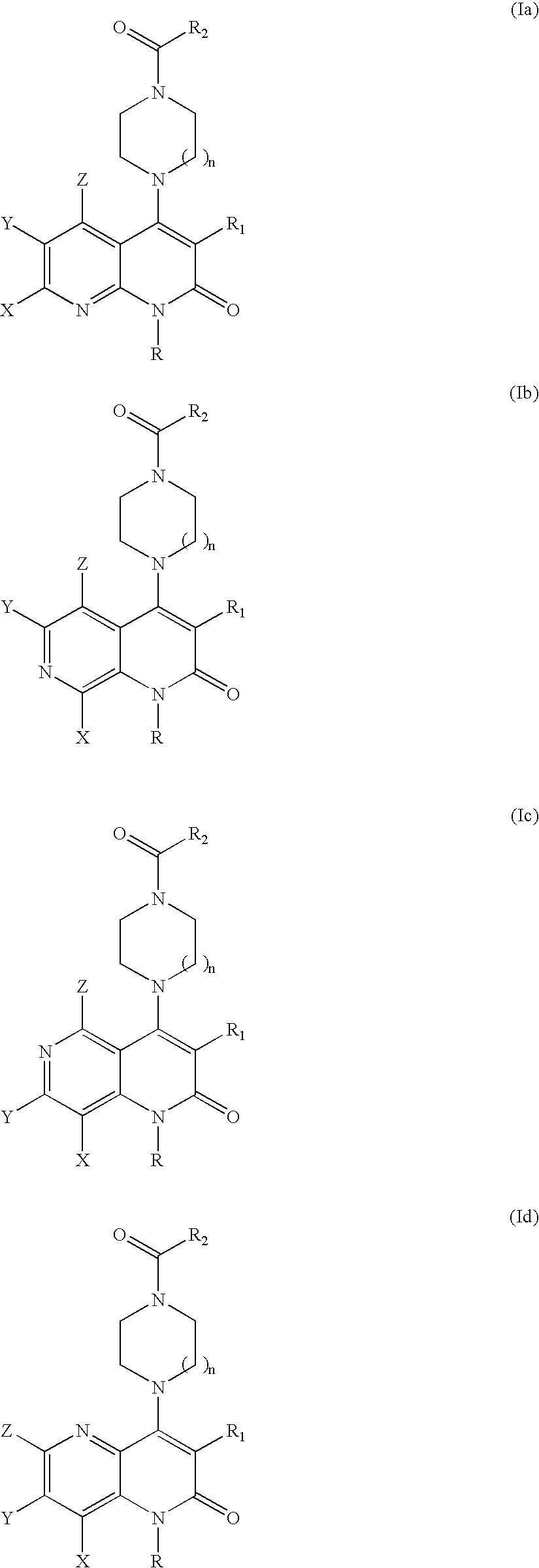
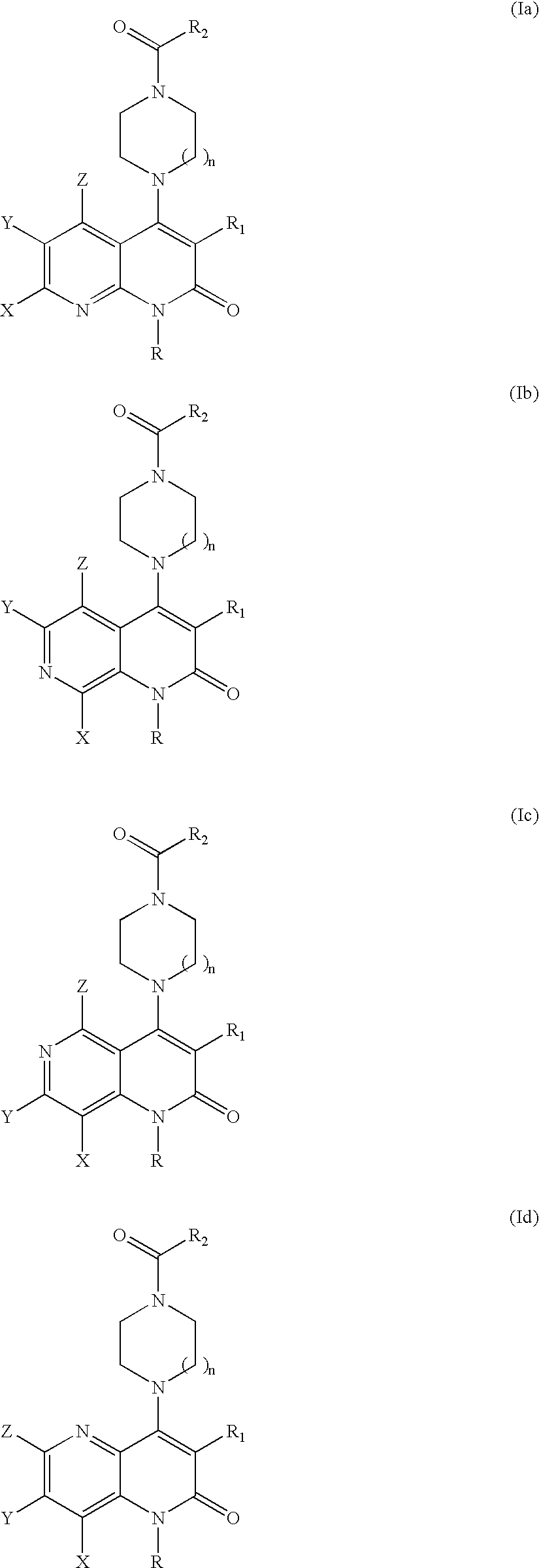
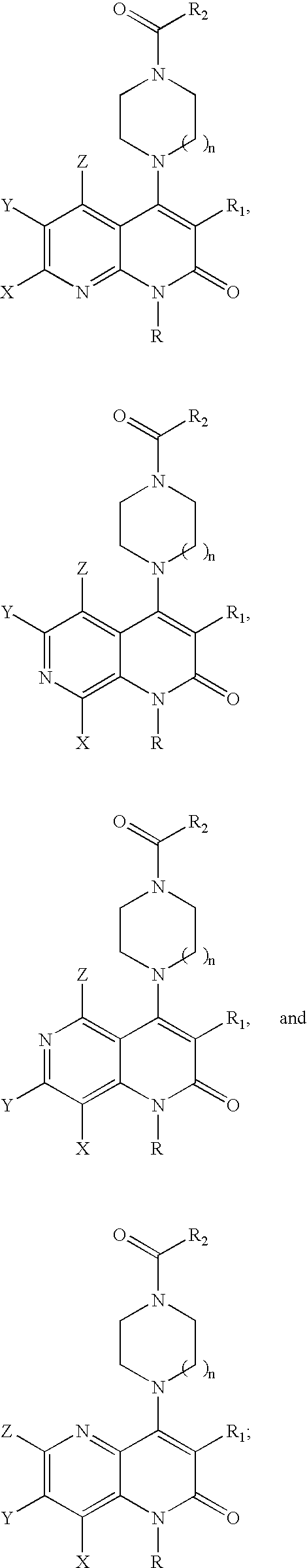
Substituted naphthyridine derivatives as inhibitors of macrophage migration inhibitory factor and their use in the treatment of human diseases
a technology of macrophage migration inhibitory factor and substituted naphthyridine, which is applied in the direction of immunological disorders, metabolism disorders, extracellular fluid disorders, etc., can solve the problems of limited ability to evaluate the inhibition of mif to its cell surface receptor, inability to detect and treat macrophage migration inhibitors, and inability to detect macrophage migration inhibitors. the effect of intracellular activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of 2-Benzylamino Nicotinic Acid (1)
[0275] Benzylamine (14 mL, 126.8 mmol) was added to a solution of chloronicotinic acid (10 g, 63.4 mmol) in pyridine and refluxed overnight. The pyridine was distilled and the residue was dissolved in 1N NaOH. The solution was diluted with water to adjust the pH to 10 to 11 and washed by dichloromethane. The aqueous phase was neutralized with cold aqueous 10% HCl solution to adjust the pH to 6 to 7. The solids formed were filtered, washed with cold water, and dried in a vacuum oven to yield 12.2 g (84%) of 2-benzylamino nicotinic acid (1) as white solids. MP: 148° C.; 1H-NMR (DMSO-d6): d 4.69 (d, J=3.6 Hz, 2H), 6.61 (dd, J=4.9, 7.7 Hz, 1H), 7.23 (m, 1H), 7.29 (m, 4H), 8.08 (dd, J=1.8, 7.0 Hz, 1H), 8.28 (dd, J=1.8, 7.0 Hz, 1H), 8.47 (br. s, 1H), 13.10 (s, 1H); EIMS: 229 (M+1), 251 (M+23).
Synthesis of 1-Benzyl-1H-pyrido[2,3-d][1,3]oxazine-2,4-dione (2)
[0276] Trichloromethyl chloroformate (2.5 mL, 21 mmol) was added slowly to a suspension...
example 2
[0457] Assays to evaluate the activity of potential MIF inhibitors are described in the following sections.
[0458] Macrophage Migration Assay
[0459] Macrophage migration is measured by using the agarose droplet assay and capillary method as described by Harrington and Stastny et al., J. Immunol. 110(3):752-759, 1973. Briefly, macrophage-containing samples are added to hematocrit tubes, 75 mm long with a 1.2 mm inner diameter. The tubes are heat-sealed and centrifuged at 100×G for 3 minutes, cut at the cell-fluid interface and imbedded in a drop of silicone grease in Sykes-Moore culture chambers. The culture chambers contain either a control protein (BSA) or samples. Migration areas are determined after 24 and 48 hours of incubation at 37° C. by tracing a projected image of the macrophage fans and measuring the areas of the migration by planimetry.
[0460] Alternatively, each well of a 96-well plate is pre-coated with one microliter of liquid 0.8% (w / v) Sea Plaque Agarose in water dis...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular mass | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com