AKT protein kinase inhibitors
a technology of akt protein and kinase, which is applied in the field of inhibitors of serine/threonine protein kinases, can solve the problems of extreme cell death (apoptosis), and the potential consequences of disease are enormous
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
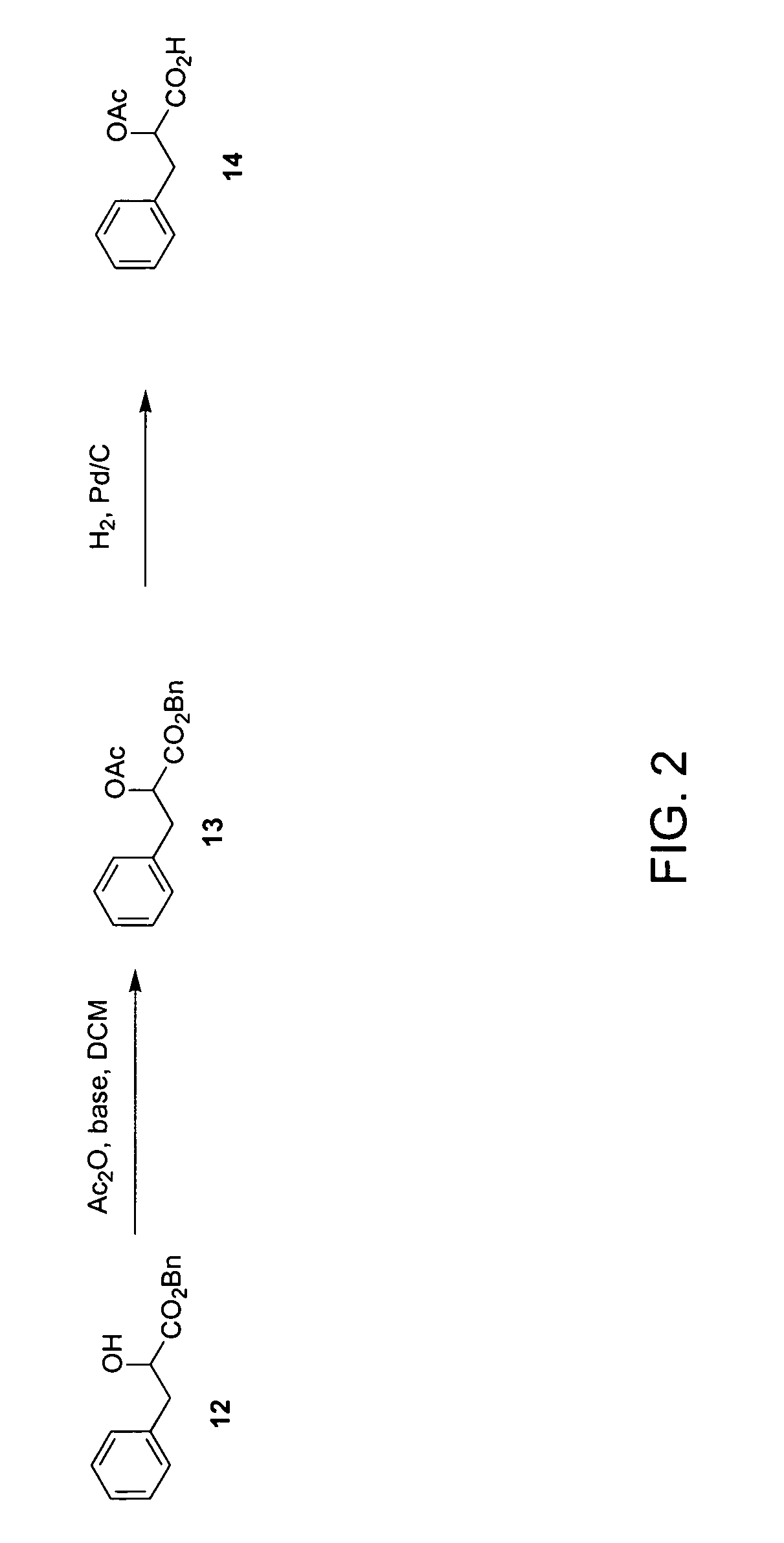
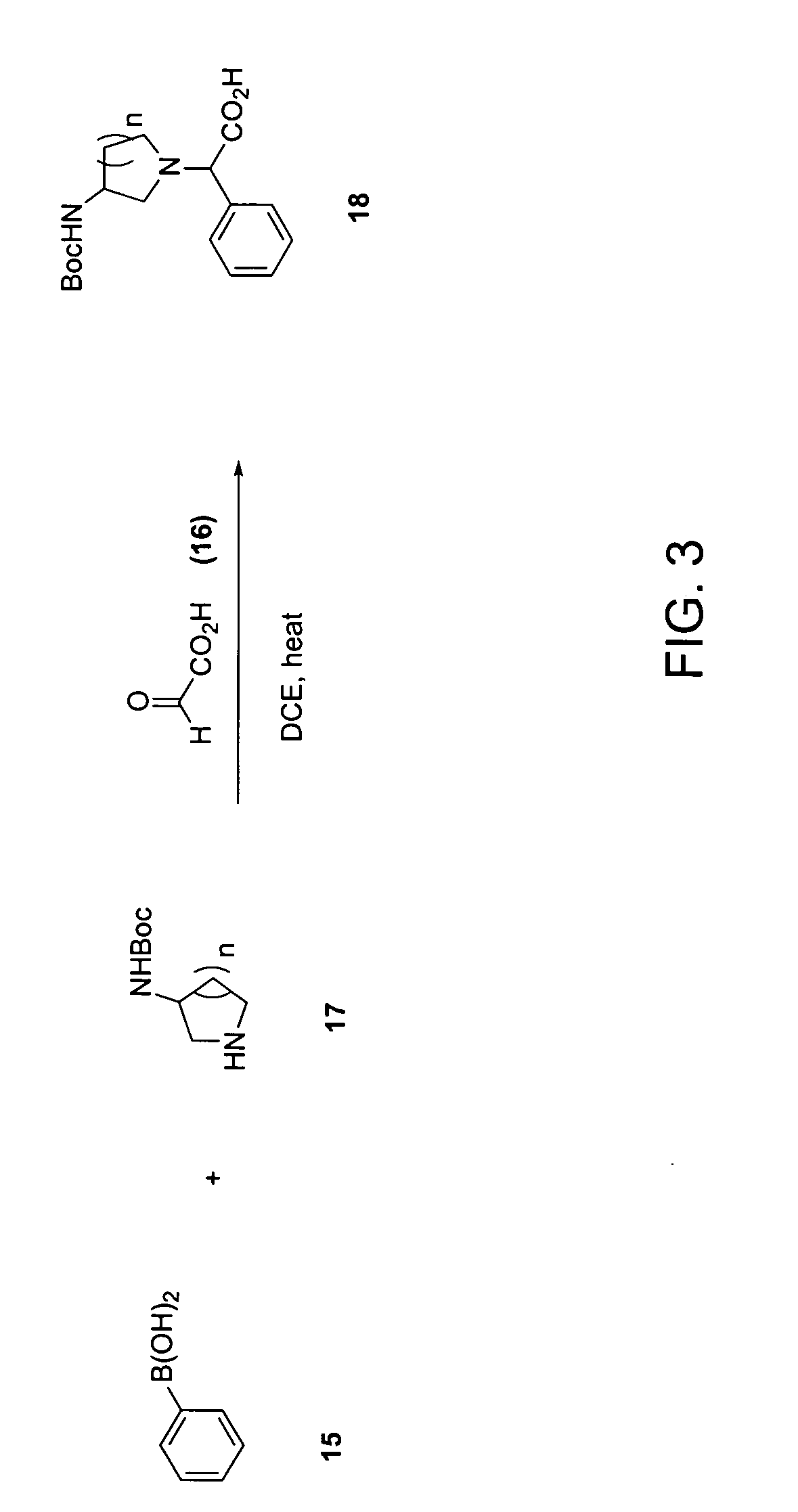
Examples
example 1a
[0303]
Preparation of 4-Piperazinylquinazoline Amino Amides
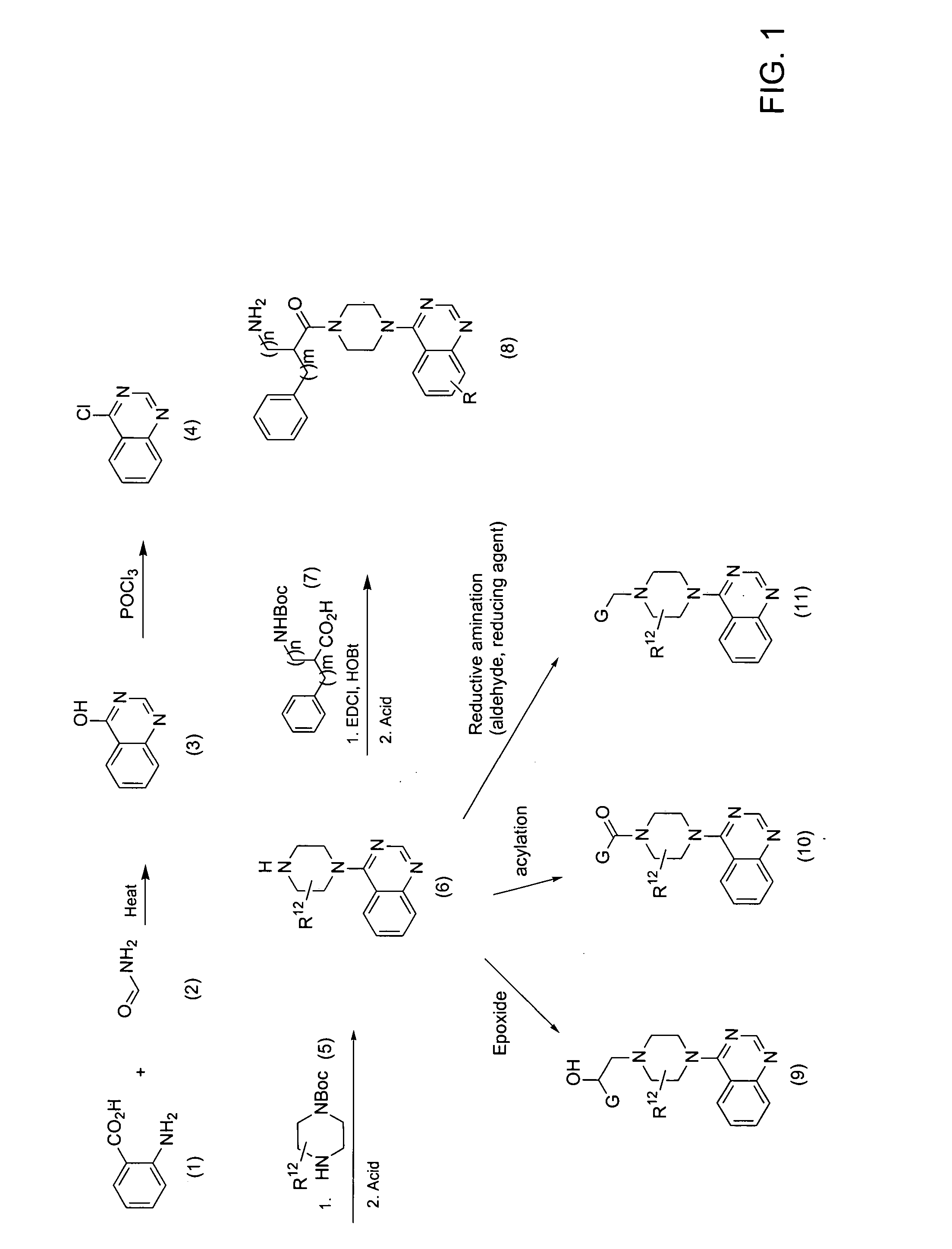
[0304] Step 1: To a solution of 4-chloroquinazoline (2.0 g, 12.2 mmol) (Tobe, Masanori, et al., Bioorg. Med. Chem. 2003, 11(3), 383) and DIEA (3.2 mL, 18.2 mmol) in 40 mL IPA was added Boc-piperazine (1.96 g, 12.81 mmol). The reaction mixture was heated to reflux and stirred for 20 hours, after which it was cooled to room temperature and concentrated by rotary evaporation. The residue was dissolved in dichloromethane (DCM) and washed with 1N NaOH. The organic layer was dried (Na2SO4), filtered, and concentrated by rotary evaporation. The resulting oil was dissolved in 25 mL dioxane, and 4M HCl / dioxane (46 mL, 182 mmol) was added dropwise. The suspension was sonicated for 2 minutes and stirred 13 hours at room temperature, after which the reaction mixture was concentrated to dryness by rotary evaporation. The resulting amine HCl salt was dissolved in 2N NaOH and extracted with DCM. The organic layer was dried (Na2SO4), filter...
example 1b
[0306] The following amino acids were introduced as Boc-protected amino acids to the 4-piperazinylquinazoline in Example 1, Step 2:
[0307] The compounds described in Examples 2-21 were prepared as described in Example 1, Step 2, using 4-piperazinylquinazoline and the appropriate amino acid shown in Example 1B.
example 2
[0308]
(2R)-2-Amino-3-phenyl-1-(4-quinazolin-4-yl-piperazin-1-yl)-propan-1-one
[0309] Rt 2.15. MS (ESI+) [M+H]+ 362.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com