Expandable medical device with beneficial agent delivery mechanism
a medical device and beneficial agent technology, applied in the field of tissue-supporting medical devices, can solve the problems of difficulty in accurately placing stents or finding and retrieving stents that subsequently become dislodged and lost in the circulatory system, struts are frequently unstable, and display a tendency to buckle, so as to improve crush strength, improve resistance to compressive forces, and minimize elastic recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
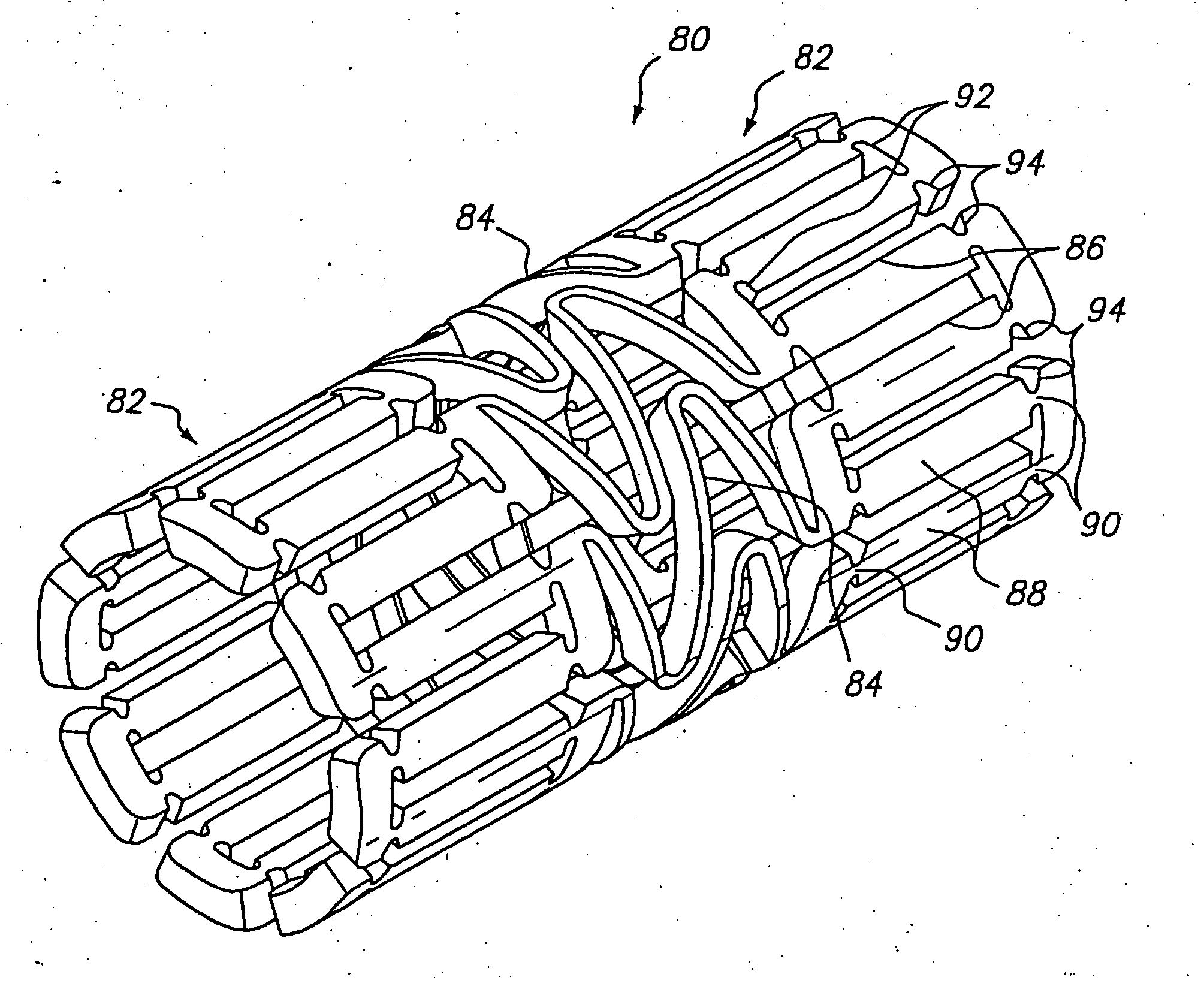
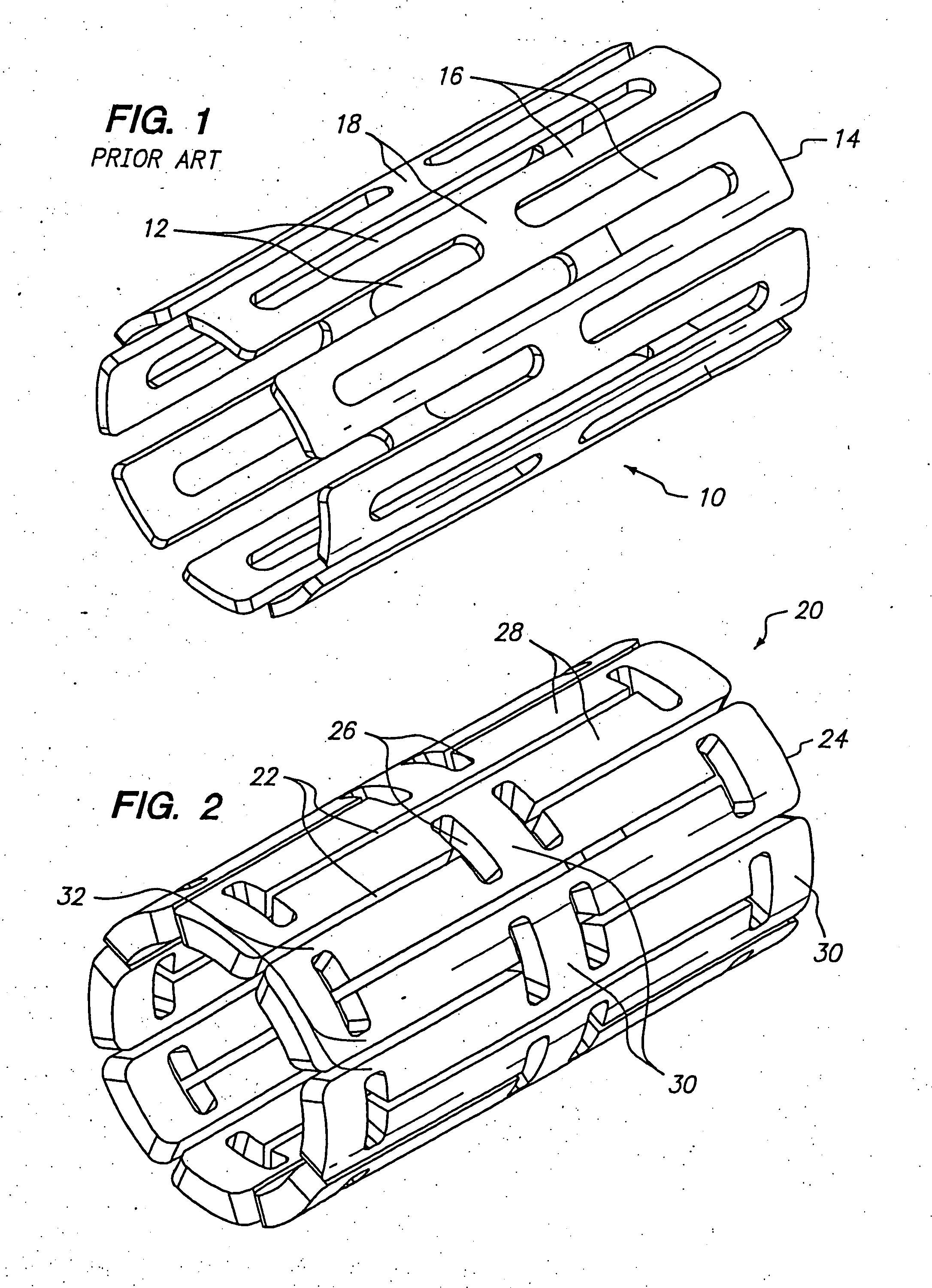
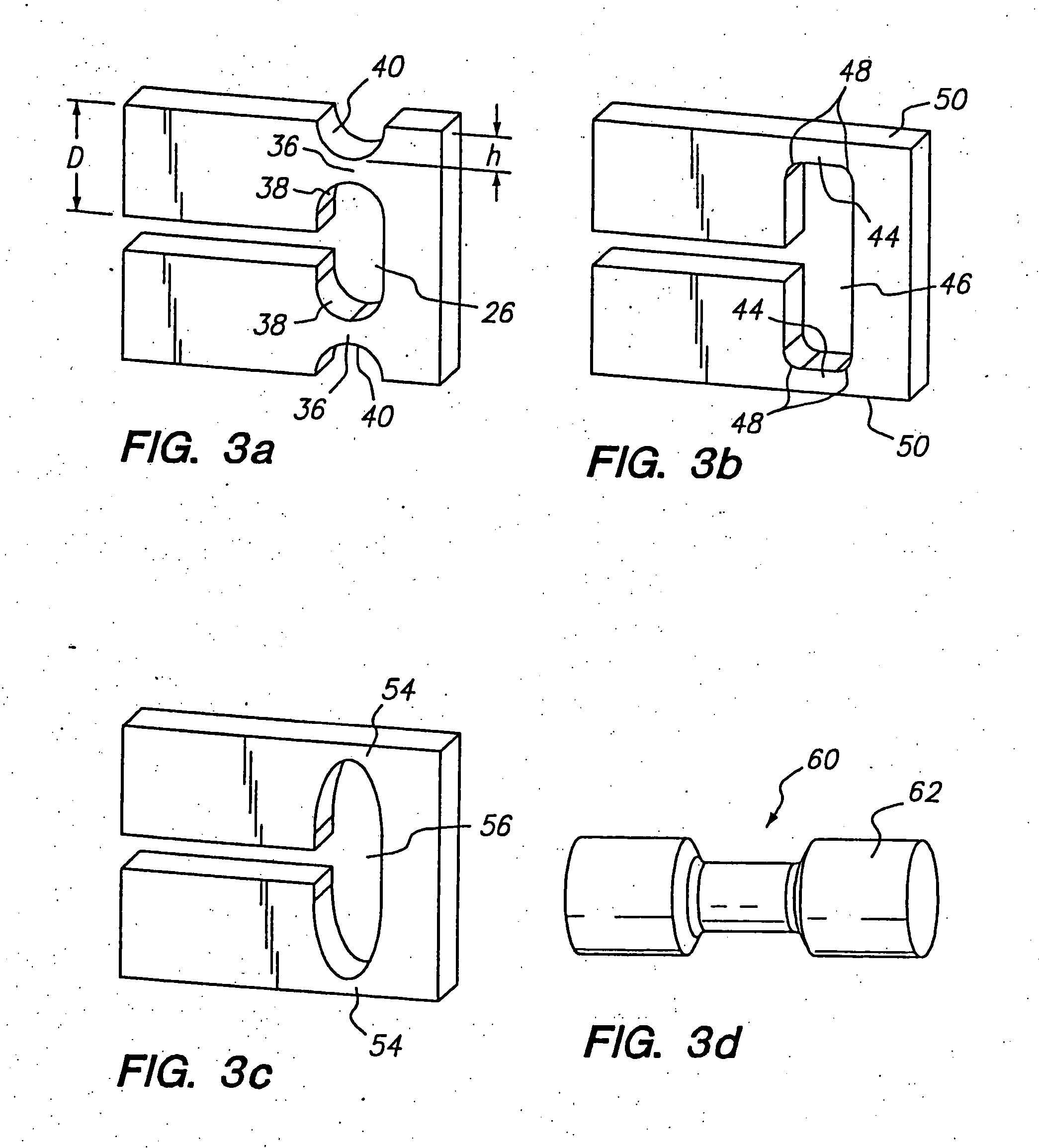
[0041]FIG. 2 shows one embodiment of an expandable tissue supporting device 20 in accordance with the present invention. The tissue supporting device 20 includes a series of axial slots 22 formed in a cylindrical tube 24. Each axial slot 22 is displaced axially from the slots in adjacent rows of slots by approximately half the slot length resulting in a staggered slot arrangement. The offset between adjacent rows of slots results in alternate rows of slots which extend to the ends of the cylindrical tube 24. At each interior end of each of the axial slots 22 a circumferential slot 26 is formed. The material between the slots 22 forms a network of axial struts 28 extending substantially parallel to an axis of the tube 24. The axial struts 28 are joined by short circumferential links 30. The circumferential links 30 are positioned at both the interior of the cylindrical tube and at the ends of the cylindrical tube. The cross section (and rectangular moment of inertia) of each of the s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com