Chimeric pro-caspases and methods of using same
a technology of pro-caspases and chimeric proteins, applied in the field of molecular biology and medicine, can solve the problems of neurodegenerative diseases that have not yet produced statistically significant gene therapy protocols, neurodegenerative diseases that have been refractory to treatment, and inborn errors of metabolism, so as to reduce the severity of pathologic conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Autoproteolytic Activation of Pro-Caspases by Oligomerization
[0123] This example demonstrates that oligomerization of pro-caspases induces proteolytic generation of mature caspase subunits and activation of their cell death activity.
Expression Plasmids
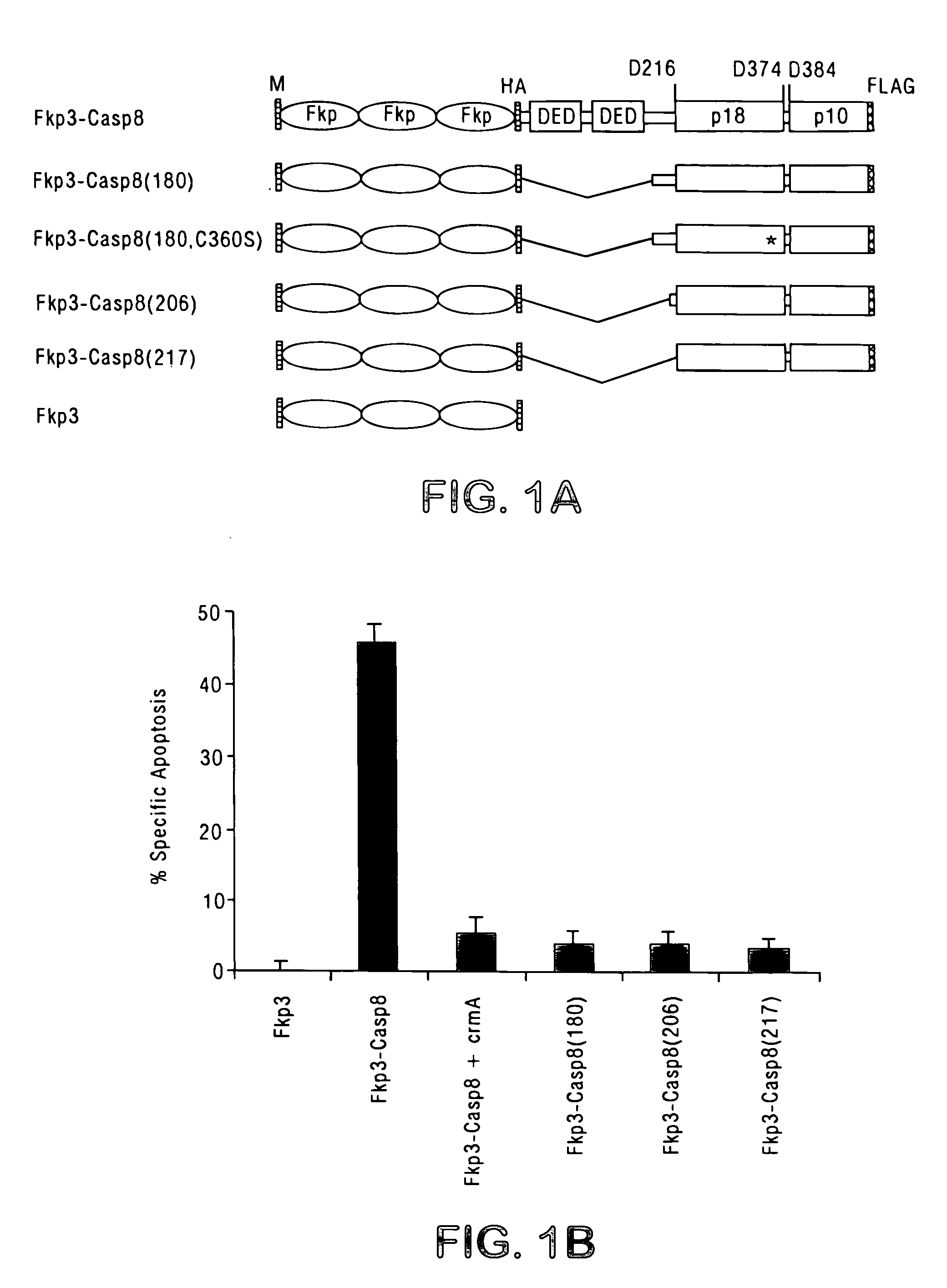
[0124] FKBP12 and FasEC fusions of caspases were constructed in pRK5. For FKBP12 fusions, a fragment containing three tandem repeats of FKBP12 with an N-terminally fused c-Src myristylation signal and a C-terminally fused HA tag was amplified by polymerase chain reaction (PCR) from pMF3E (Spencer et al., Science 262:1019-1024 (1993), which is incorporated herein by reference), digested with EcoRI and BamHI, and cloned into pRK5, yielding pFkp3-HA. DNA fragments containing full-length and deletion mutants of human pro-caspase-8 with a C-terminal FLAG tag were digested with BamHI and HindIII and cloned into pFkp3-HA. pFkp3 was made by deleting the SalI / XhoI fragment of caspase-8(180) in pFkp3-Casp8(180).
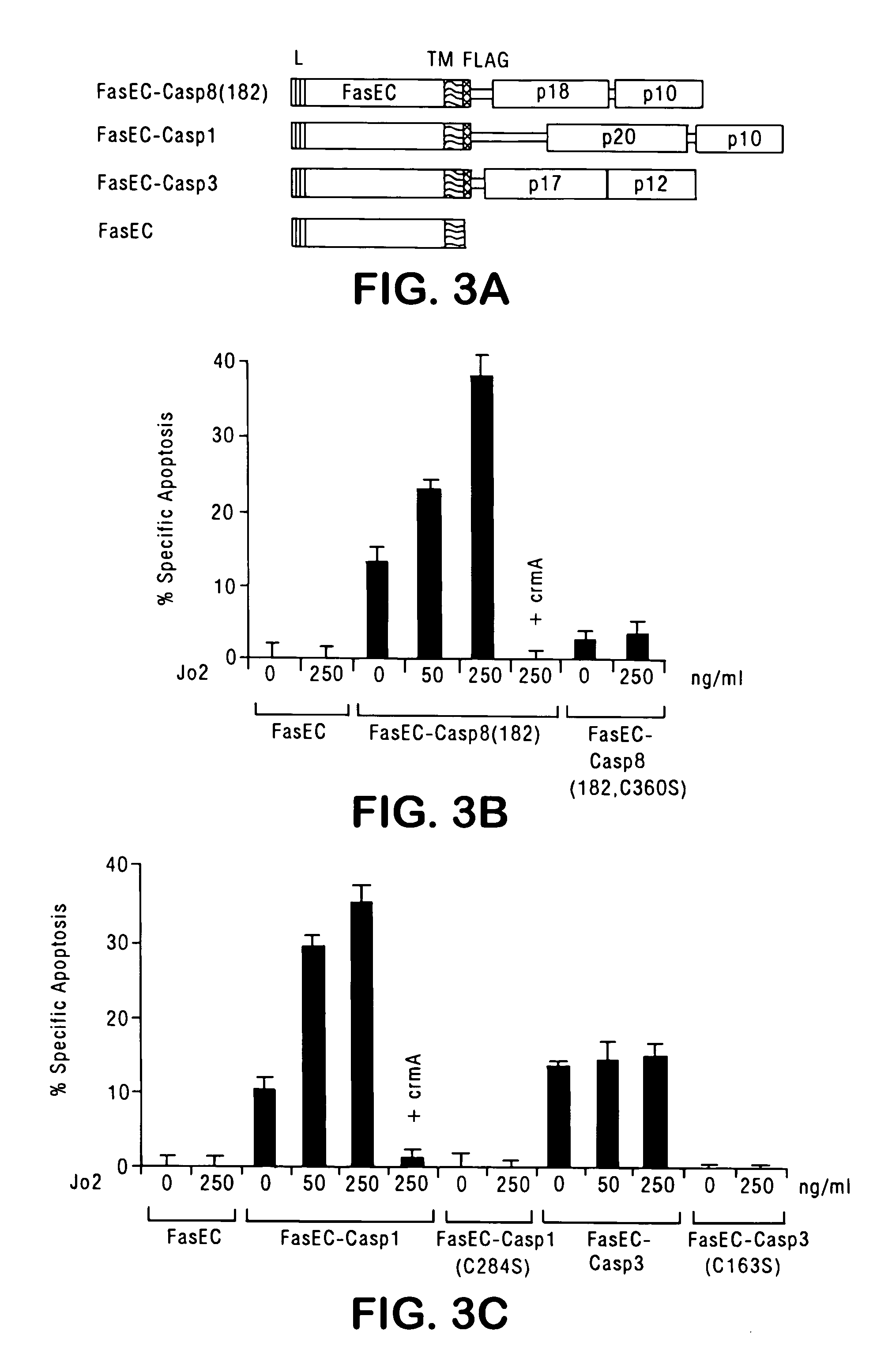
[0125] Murine FasEC (residues...
example ii
Preparation and Characterization of Chimeric Pro-Caspase
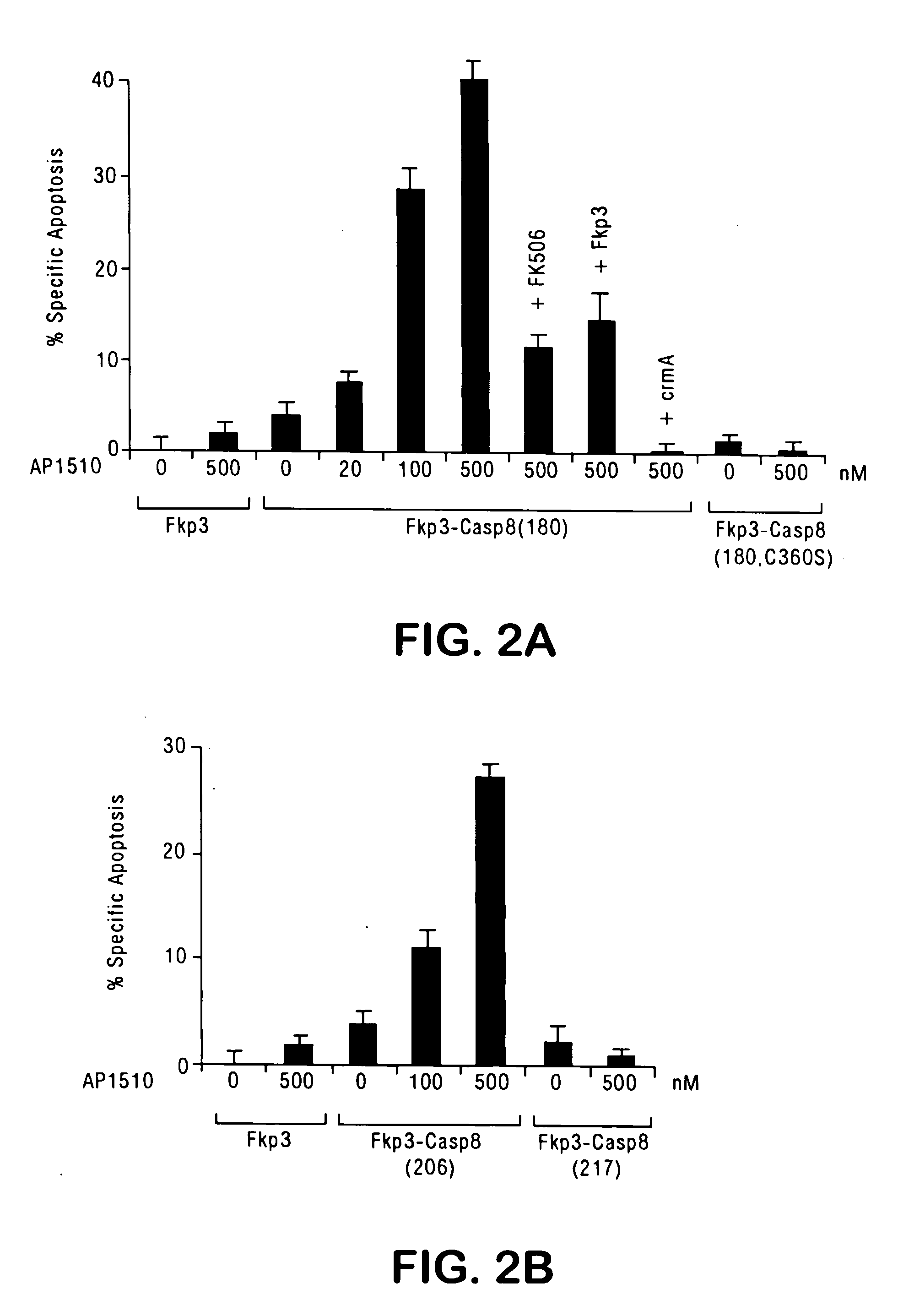
[0142] This example describes the construction and characterization of viral vectors expressing an FKBP-pro-caspase fusion polypeptide that can effectively induce apoptosis upon oligomerization, but that otherwise demonstrates minimal autotoxicity.
[0143] For oligomerization of caspases to be an effective suicide system, the caspase should have maximal cytotoxicity in the presence of a dimerizing agent, but minimal cytotoxicity in the absence of the dimerizer. The human FKBP protein is used for the oligomerization motif. The efficacy of FKBP dimerization has been demonstrated in various cells for diverse biological processes (Spencer et al., supra, 1993; Amara et al., supra, 1997; Freiberg et al., J. Invest. Dermatol. 108:215-219 (1997); Freiberg et al., J. Biol. Chem. 271:31666-31669 (1996); Holsinger et al., Proc. Natl. Acad. Sci., USA 92:9810-9814 (1995)). To minimize the binding of dimeric ligands to endogenous FKBP, the F...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pharmaceutical composition | aaaaa | aaaaa |
| cell cycle time | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com