Methods for designing specific ion channel blockers
a technology of ion channel blocker and design method, which is applied in the direction of immunoglobulins, peptides, antibody medical ingredients, etc., can solve the problems of lack of specificity, lack of functional redundancy, complicated results interpretation, etc., and achieves rapid and effective means
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Suppression of Kv1.2 Current by Kv1.2-BA Antibody
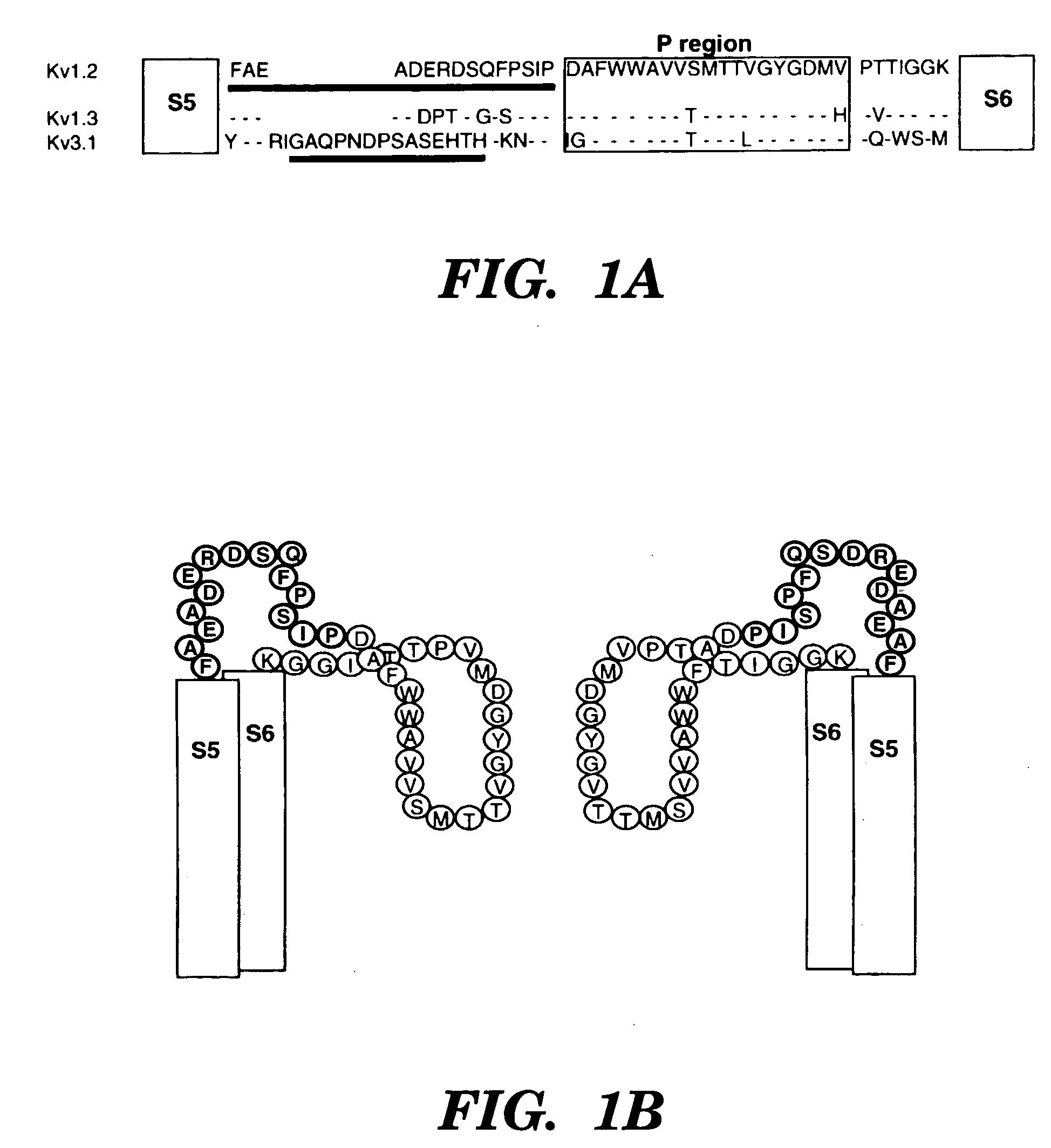
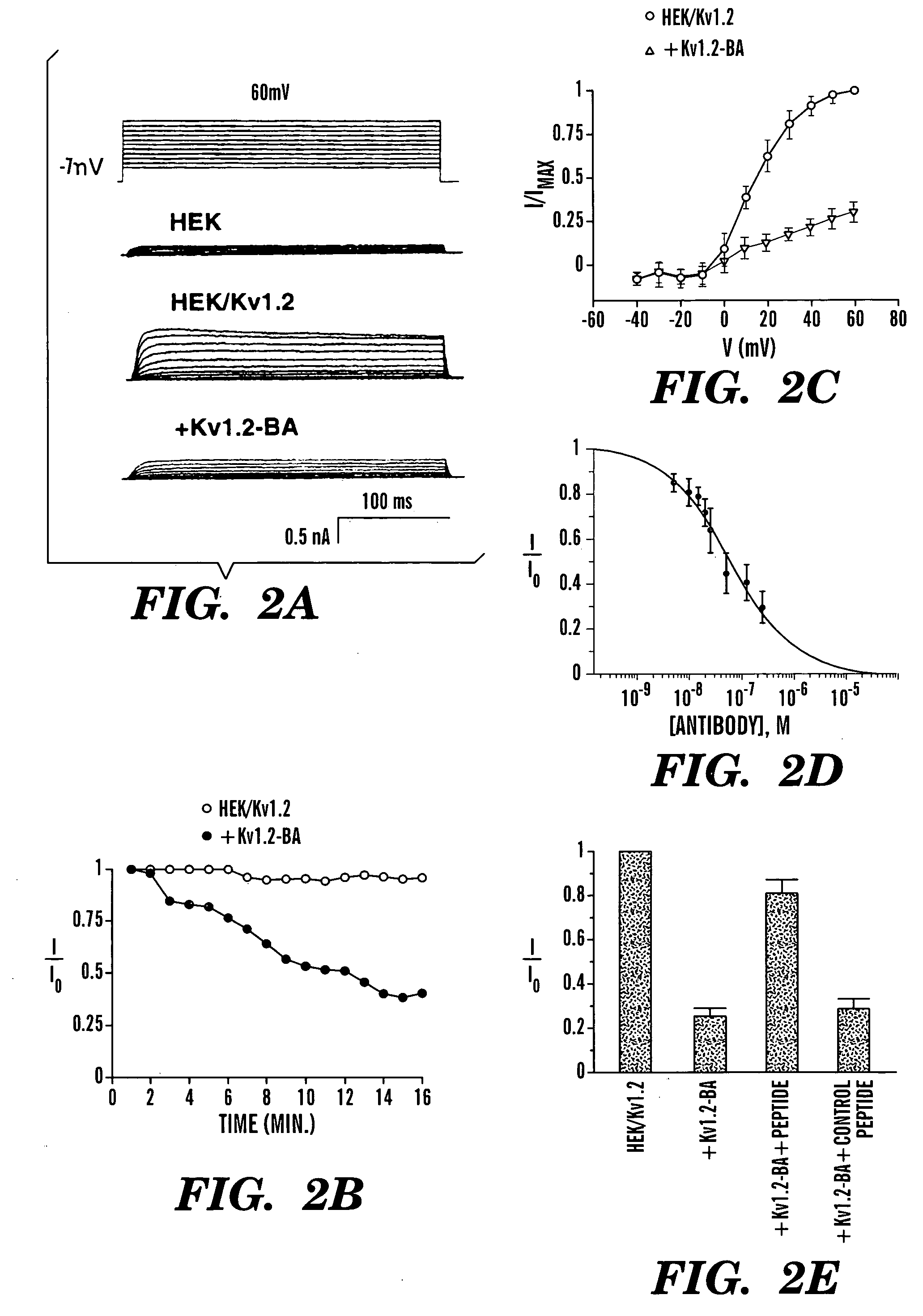
[0055] To test whether antibodies specific for peptides around the pore-regions of individual ion channels can be used as a channel blocker, the blocking ability of an affinity-purified polyclonal antibody generated against a 15 amino-acid peptide of a delayed-rectifier potassium channel Kv1.2 (Huang, X. Y. et al., Cell 75:1145-1156 (1993)) (FIG. 1A) was examined. This sequence identified as SEQ ID NO:1:
Phe Ala Glu Ala Asp Glu Arg Asp Ser Gln Phe Pro Ser Ile Pro1 5 10 15
located between the S5 transmembrane and the pore-forming (P) region, is very likely part of the external vestibule (or the outer mouth) of the channel protein (FIG. 1B) (Lu, Q. et al., Science 268:304-307 (1995); Hidalgo, P. et al., Science 268:307-310 (1995); Aiyar, J. et al., Neuron 15:1169-1181 (1995), which are hereby incorporated by reference). Kv1.2 was stably expressed in a mammalian cell line, HEK-293 cells ...
example 2
[0056] To ensure that the Kv1.2-BA antibody does indeed bind to the external region of Kv1.2 channel protein and that the blocking effect is due to the binding of the Kv1.2-BA antibody to the channel protein, Kv1.2-BA antibody was preincubated with the immunogenic peptide that was used to generate Kv1.2-BA. If blocking is due to binding of the antibody to the peptide sequence in the external vestibule of the channel protein, preincubation with the peptide should prevent the inhibition. As shown in FIG. 2E, addition of 250 nM Kv1.2-BA after preincubation with the immunogenic peptide only produced about 25% inhibition. Preincubation with a control peptide did not inhibit the Kv1.2-BA-induced suppression of Kv 1.2 currents (FIG. 2E). Thus, the majority of the inhibition by Kv1.2-BA on Kv1.2 currents is due to specific finding of Kv1.2 to the particular peptide sequence around the pore region of Kv1.2 channels. The residual about 25% inhibition is likely due to either nonspecific blocki...
example 3
[0057] No Effect of Kv1.2 Current by a Control Antibody Kv1-NA
[0058] To further exclude the possibility that the Kv1.2 BA blocking effects were non-specific and that any antibody added outside cells somehow interferes the channel function, the effects on the Kv1.2 currents by another affinity-purified polyclonal antibody (Kv1-NA) was examined (FIG. 3A and 3B). Kv1-NA was generated against a peptide having SEQ ID NO:2:
Asp Pro Leu Arg Asn Glu Tyr Phe Phe Asp Arg Asn Arg Pro Ser1 5 10 15
from the intracellular N-terminus of Kv1.2, which is identical in all members of the Kv1 family (FIG. 1D). Addition of this control antibody Kv1-NA had no effect at low concentrations (100 nM) on the Kv1.2 currents (FIG. 3B). These results indicate that the effect of antibody Kv1.2-BA is specific.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Ion transport number | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com