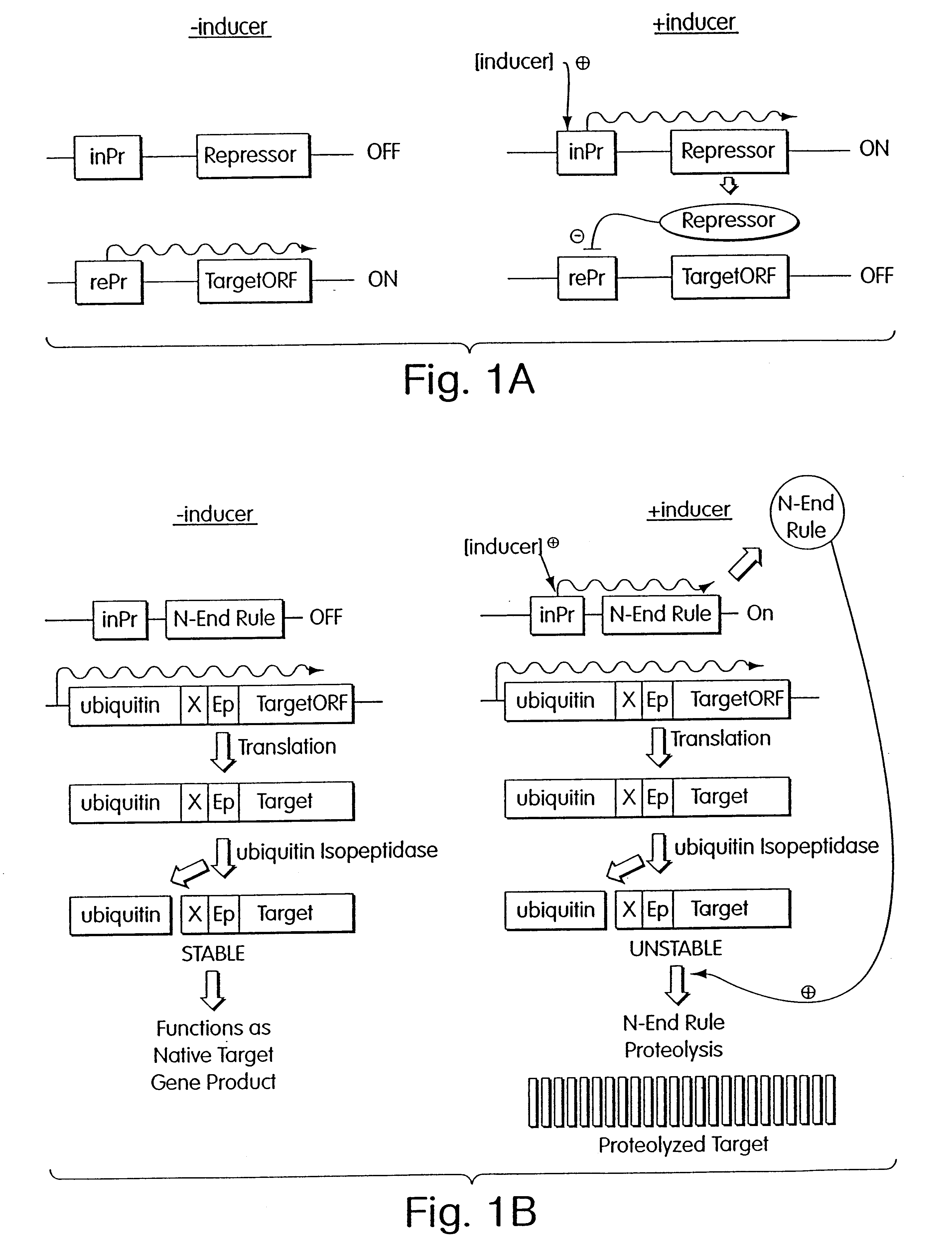
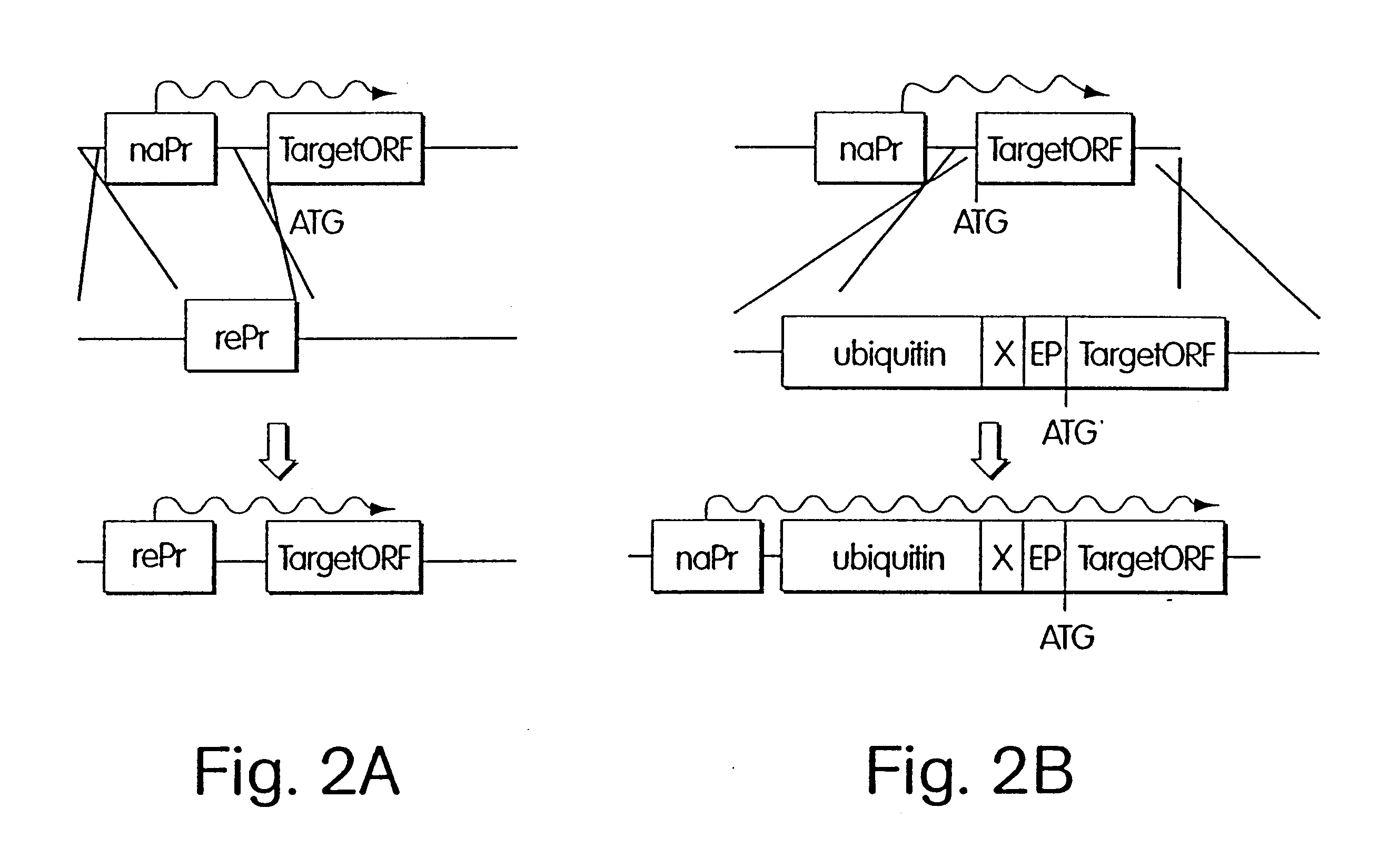
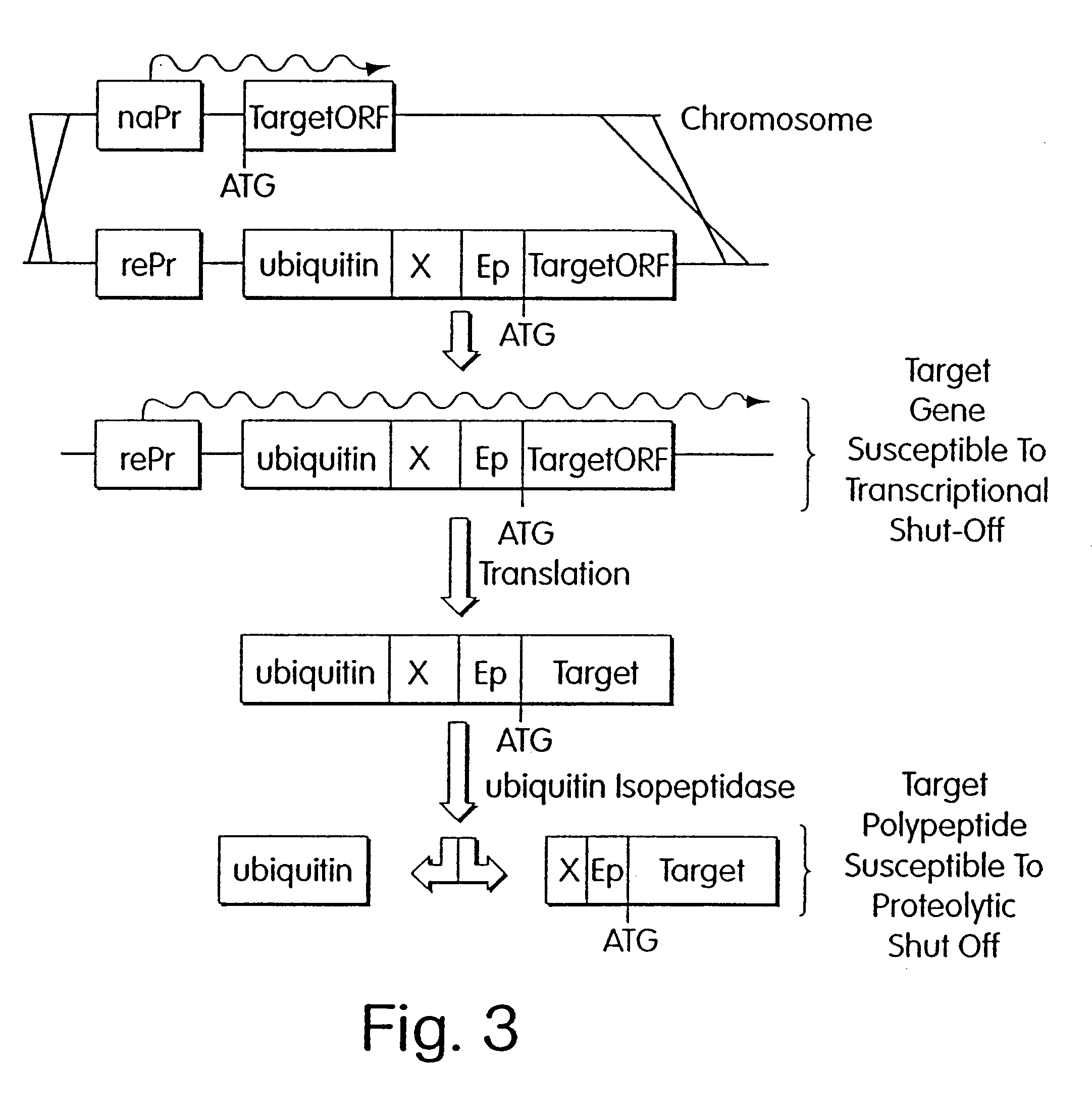
Inducible methods for repressing gene function
a technology of gene function and inducible methods, which is applied in the field of inducible methods for suppressing gene function, can solve the problems of loss of function of encoded gene product, many limitations in the genetic approach, and exceedingly difficult study of essential genes, required for cell viability, and purely genetic approach
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
5.1 Example 1
Use of Double Shutoff Yeast Strain Containing Copper-Inducible Yeast Alleles of Both ROX1 And UBR1 to Determine that TBP-Associated Factors are not Generally Required for Transcriptional Activation in Yeast
Materials and Methods
The parent strain ZMY60, containing copper-inducible alleles of UBR1 and ROX1, was created as follows. A cassette containing the copper-inducible derivative of the HIS3 promoter (Klein, C and K. Struhl (1994) Science 266: pp. 280-282) and 2 kb of upstream flanking sequence was inserted at the initial ATG of a plasmid-borne genomic fragment of ROX1 to create the URA3 integrating plasmid ZM195. The same cassette was inserted at the initial ATG of UBR1 to create ZM197. Both of these copper-driven alleles were introduced into yeast strain KY114 (Iyer, V and K. Struhl (1995) Mol. Cell. Biol. 116: pp. 7069-7086) in successive two-step gene replacements. To create TAF disruption molecules, another cassette comprising an inframe fusion of ubiquitin, argin...
example 2
5.2 Example 2
The following example demonstrates how any given target gene can be configured for the double shutoff system in Saccharomyces cervisiae. The system requires two basic components: first, a parent strain containing copper-inducible alleles of both ROX1 and UBR1; and second, a short 5' fragment of the target gene of interest fused in frame to a ubiquitin-arginine-lacI-HA ("URLF") cassette and driven by the ANB1 promoter.
The strain ZMY60 has the genotype: MAT ay, ACE-UBR1, Ace-ROX1, trpl-D1, ura3-52Z LEU2, HIS3, ade2-101 in a KY114 background. To generate a parent strain in a different background, one utilizes the URA3 integrating plasmids ZM195 and AM197, which must be used in successive two-step gene replacements to generate a parent strain containing copper-inducible ROX1 and UBR1. To create double-shutoff parent strain, one integrates ZM195 into a desired strain with AflII. The URA3 marker is the loopout on FOA (5-fluorotica acid). One would then check for correct loopo...
example 3
5.3 Example 3
DKO strains and constructs of TAF19 (strain ZMY67), TAF60 (ZMY66), TAF90 (ZMY68), TAF 130 (ZMY69, TBP, AND TFIIB (ZMY71).
Strain ZMY60 (the DKO parent strain)
Useful Saccharomyces cervisiae Strains
Parent Strains
ZMY59 (Ace-UBR1 only strain)
ZMY61 (Ace-ROX1 only strain)
ZMY103 (ZMY60 his3.DELTA.200)
ZMY117 (ZMY60 leu2::PET56)
ZMY118 (ZMY60 his3.DELTA.200, leu2::PET56)
Shutoff Strains
PUM
| Property | Measurement | Unit |
|---|---|---|
| Degradation properties | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com