Vacuum package system and method
a vacuum package and vacuum technology, applied in the field of vacuum package system and method, can solve the problems of high sterility of containers, low or non-pyrogenic level, low level of non-viable particulate matter, etc., and achieve the effect of reducing the number of contaminated containers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] Certain terminology is used in the following description for convenience only and is not limiting. The words “right,”“lower” and “upper” designate directions in the drawings to which reference is made. The words “inwardly” and “outwardly” refer to directions toward and away from, respectively, the geometric center of the vacuum package system and designated parts thereof. The terminology includes the words specifically mentioned, derivatives thereof and words of similar import. Additionally, the word “a” as used in the specification means “at least one.”
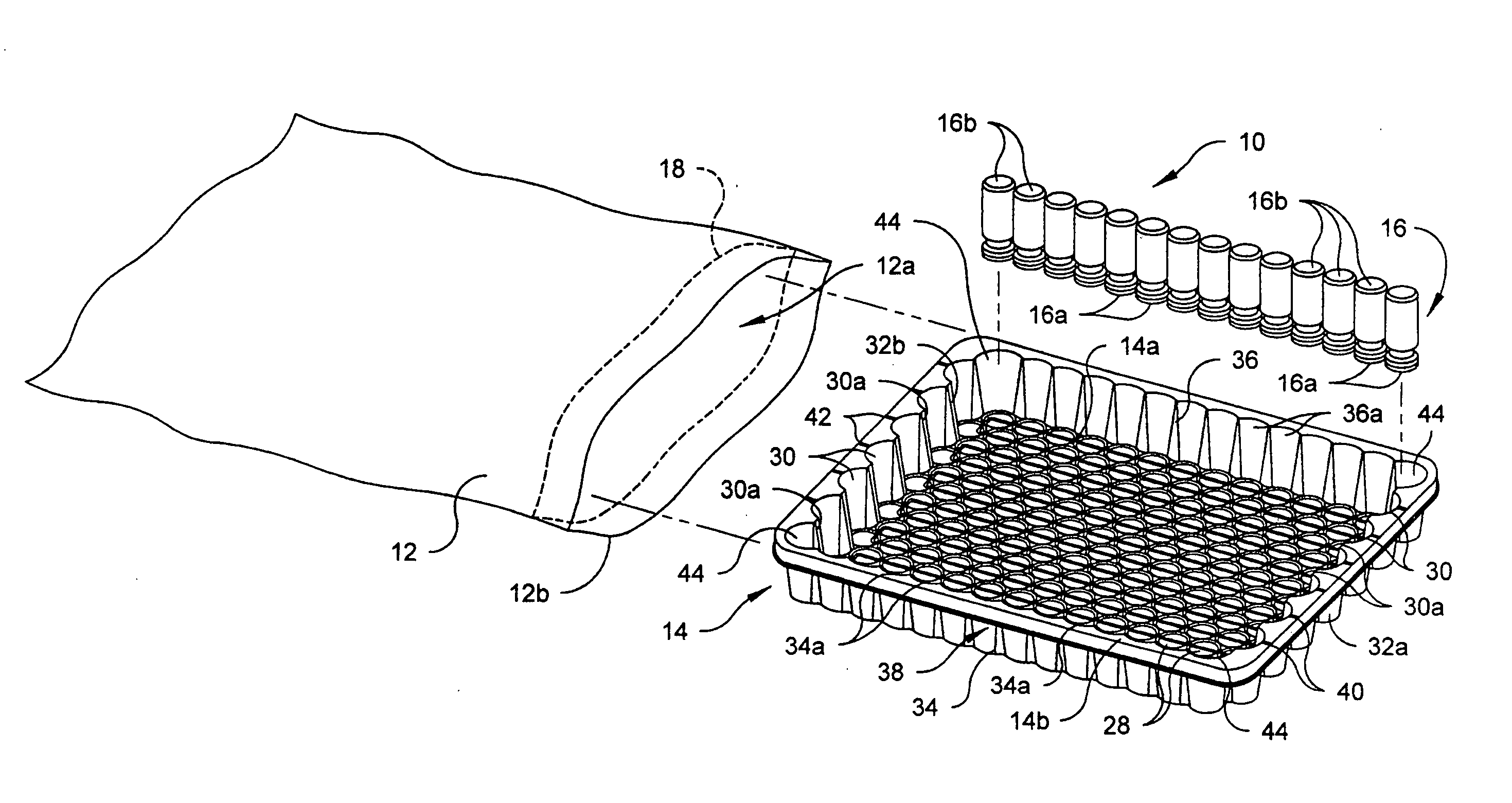
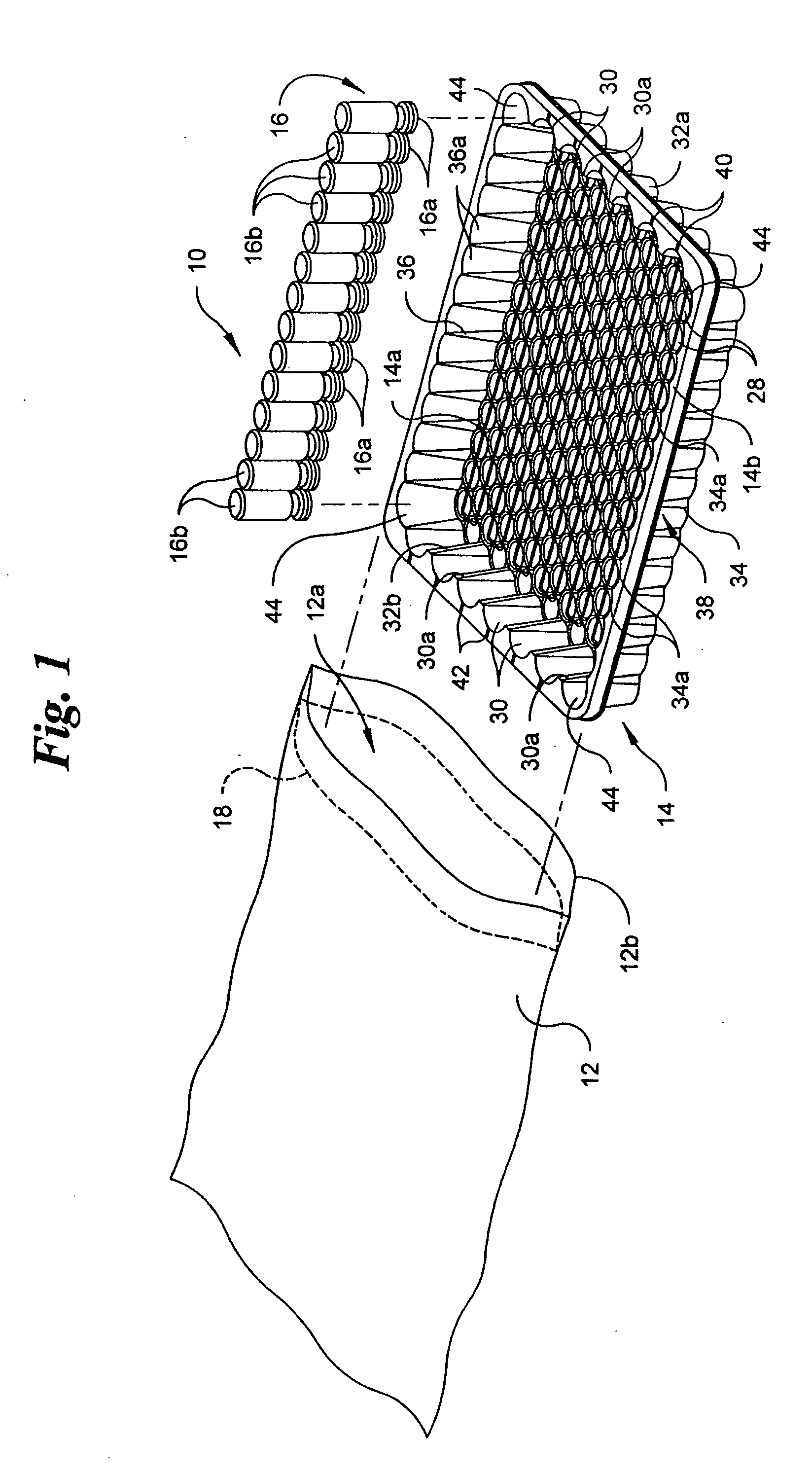
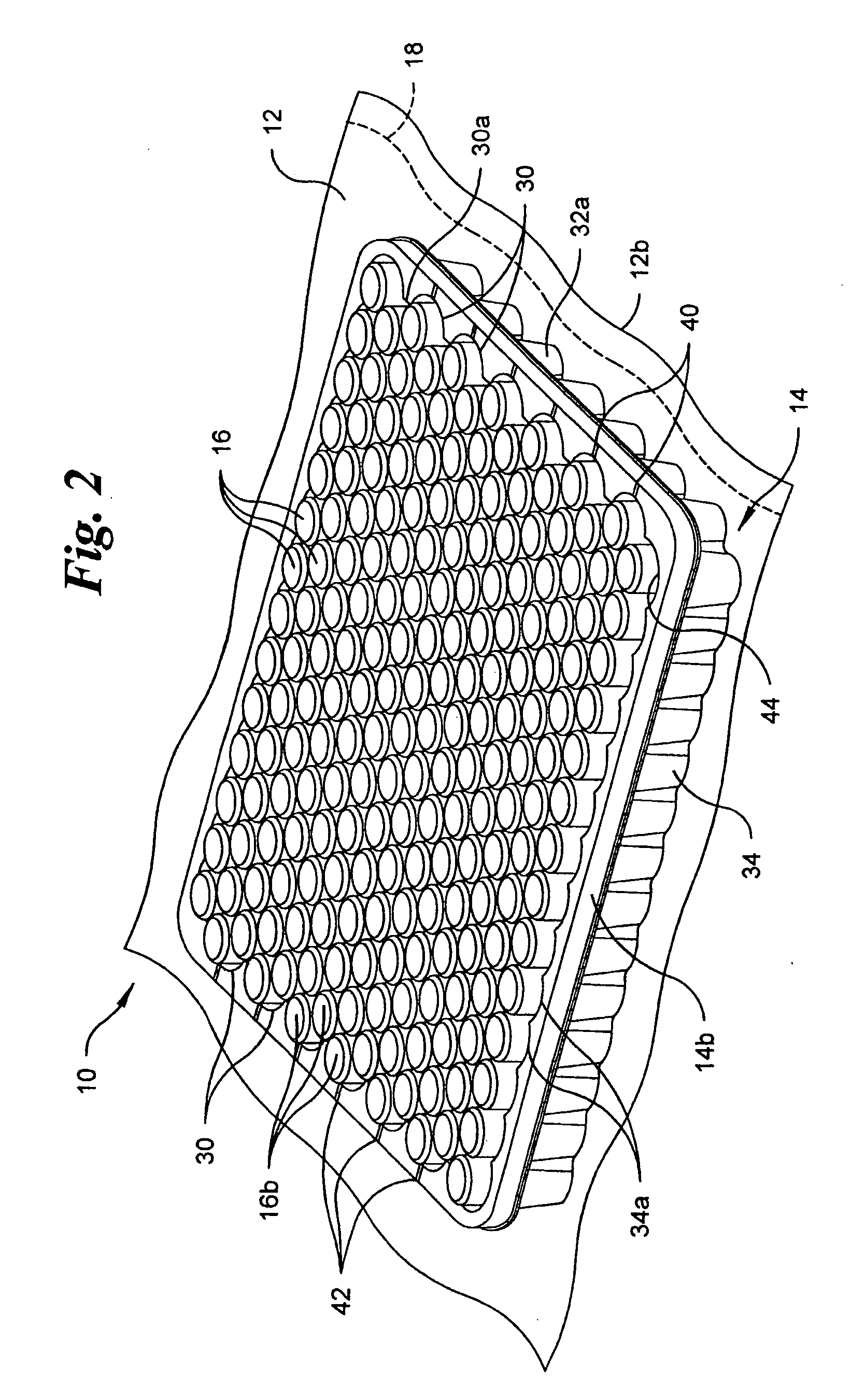
[0020] Referring to the drawings in detail, wherein like numerals indicate like elements throughout, there is shown in FIGS. 1-5 and 8 a first preferred embodiment of a vacuum packaging system, generally designated 10, for transporting a plurality of medical containers 16. The vacuum packaging system 10 includes an air impervious film 12, a tray 14 and the plurality of medical containers 16. In the first preferred embodiment, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| flexible | aaaaa | aaaaa |
| atmospheric pressure | aaaaa | aaaaa |
| vacuum | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com