Implantable spinal device revision system
a spinal device and implantable technology, applied in the field of implantable spinal device revision system, can solve the problems of future disc degeneration, addition of problems and/or complications, and joint failure of the spin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
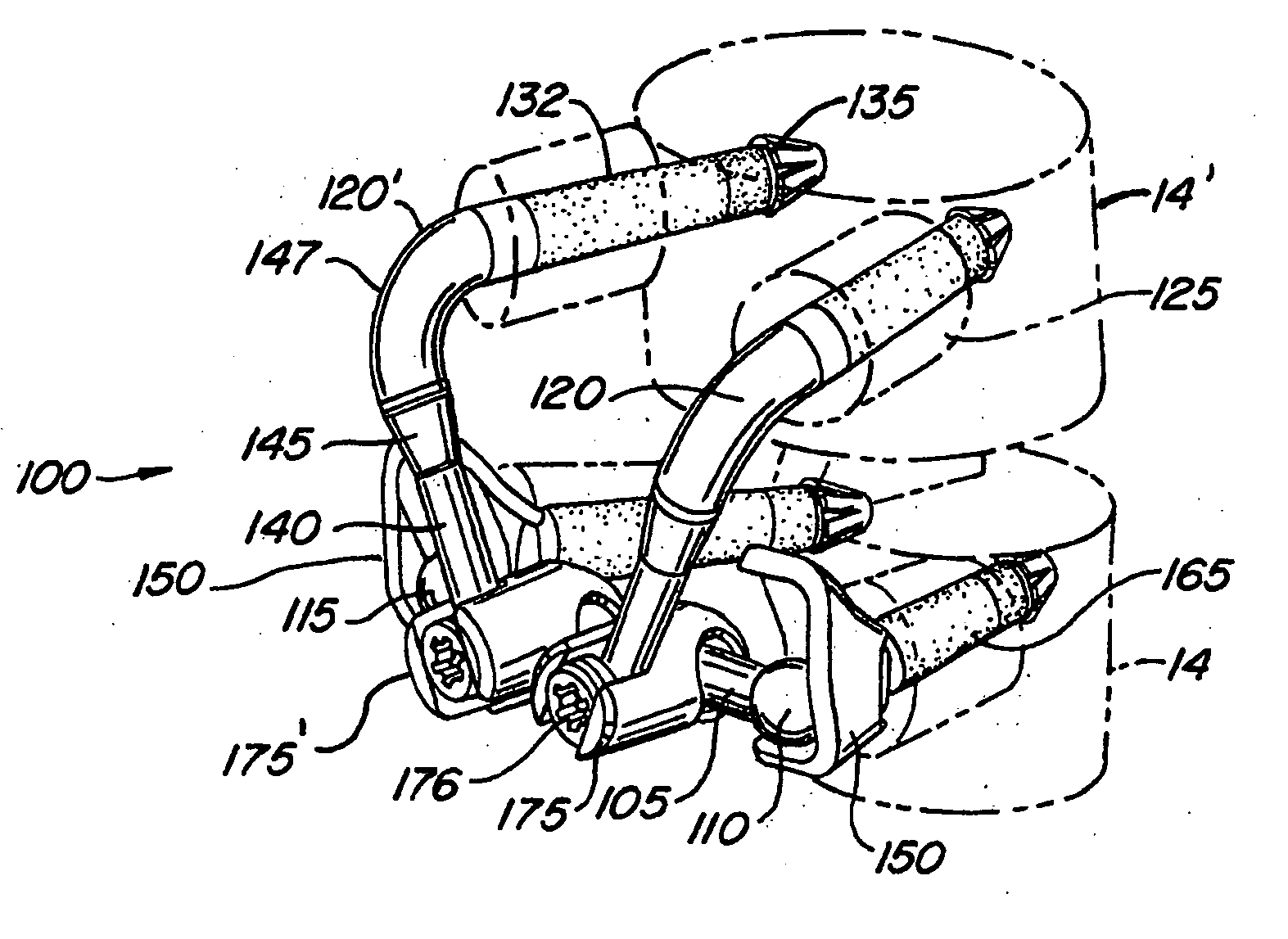
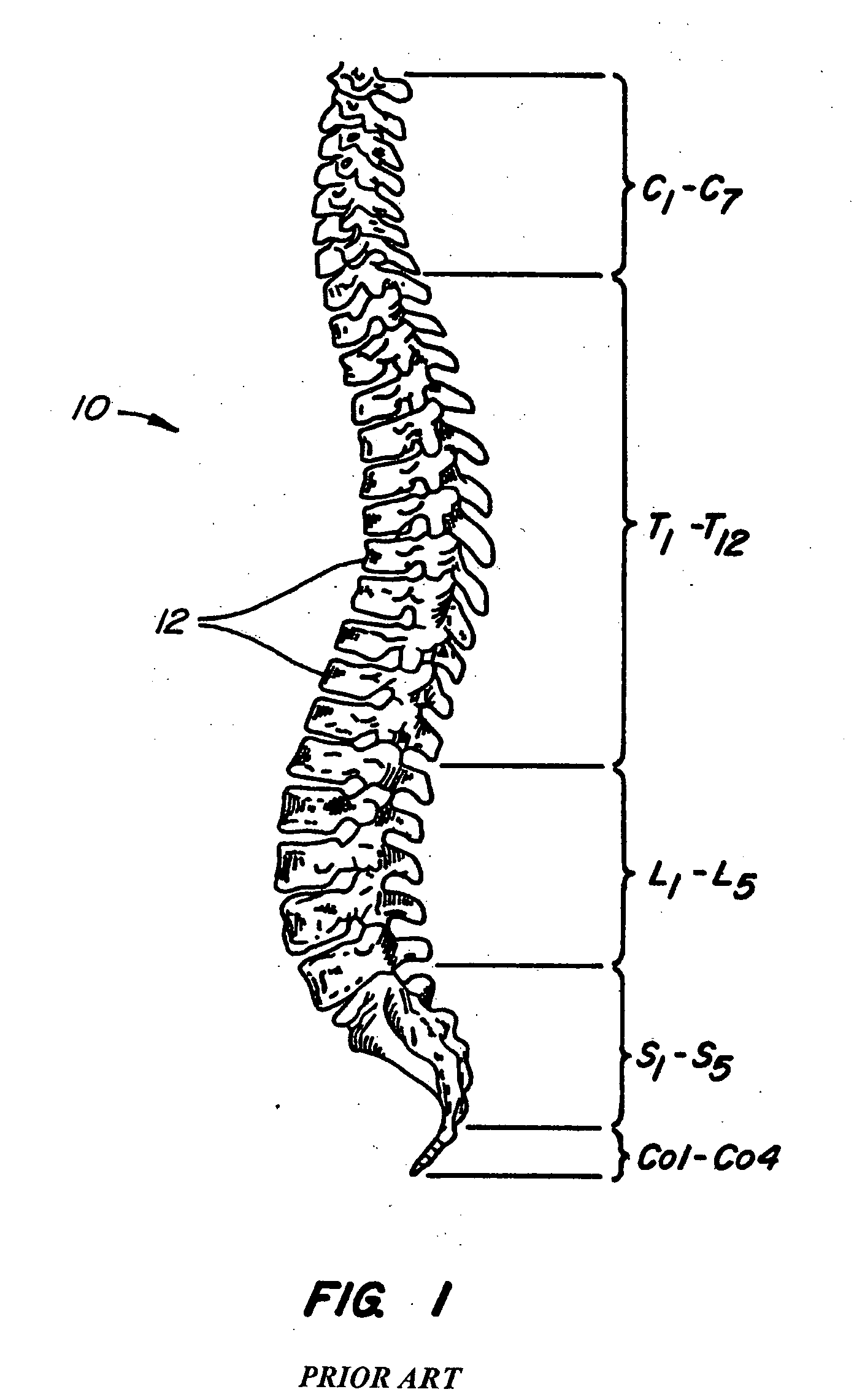
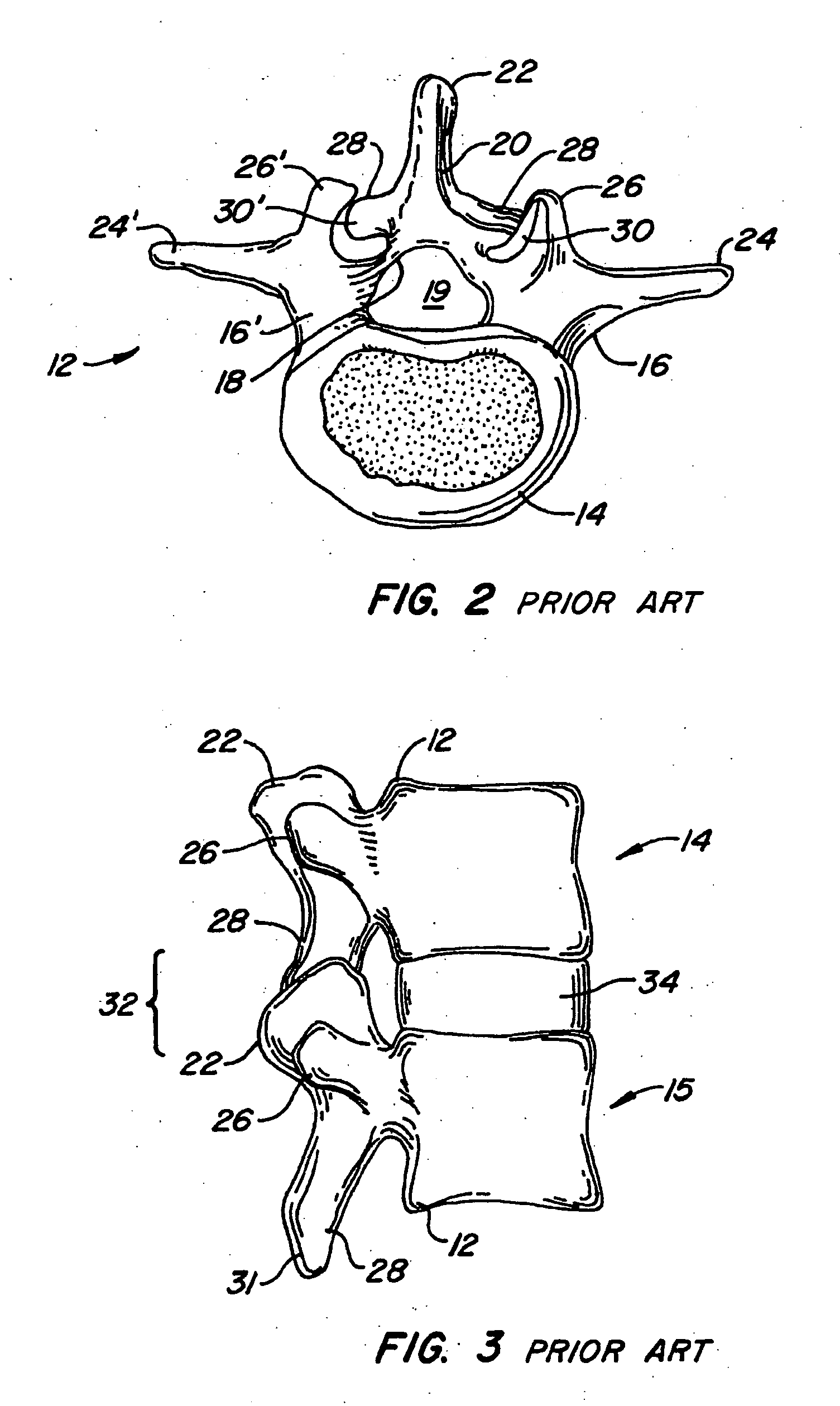
[0077] The invention relates generally to implantable devices, apparatus or mechanisms that are suitable for implantation within a human body to restore, augment, and / or replace soft tissue and connective tissue, including bone and cartilage, and systems for treating spinal pathologies. In some instances the implantable devices can include devices designed to replace missing, removed or resected body parts or structure. The implantable devices, apparatus or mechanisms are configured such that the devices can be formed from parts, elements or components which alone or in combination comprise the device. The implantable devices can also be configured such that one or more elements or components are formed integrally to achieve a desired physiological, operational or functional result such that the components complete the device. Functional results can include the surgical restoration and functional power of a joint, controlling, limiting or altering the functional power of a joint, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com