Medicament carriers and methods of using same
a technology of medicament carriers and carriers, which is applied in the field of medicament storage and dispensing, can solve the problems of reducing the likelihood of errors in the administration of medicaments in health care facilities, subject devices suffer from a number of limitations, and no accurate way to inventory medicaments
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0031] As used herein, a “medicament” may include one or more of an individual, unit-of-issue dose of prescription and / or non-prescription medications, medical supplies, pharmaceuticals, nutraceuticals, injectibles, medical devices, diagnostic materials and other therapeutic products. A medicament may, where applicable, be in liquid, solid or gaseous form. Specific examples of medicaments may include, without limitation, suppositories, pre-filled syringes, inhalers, lotions, suspensions, blood testing strips, pills, tablets and caplets.
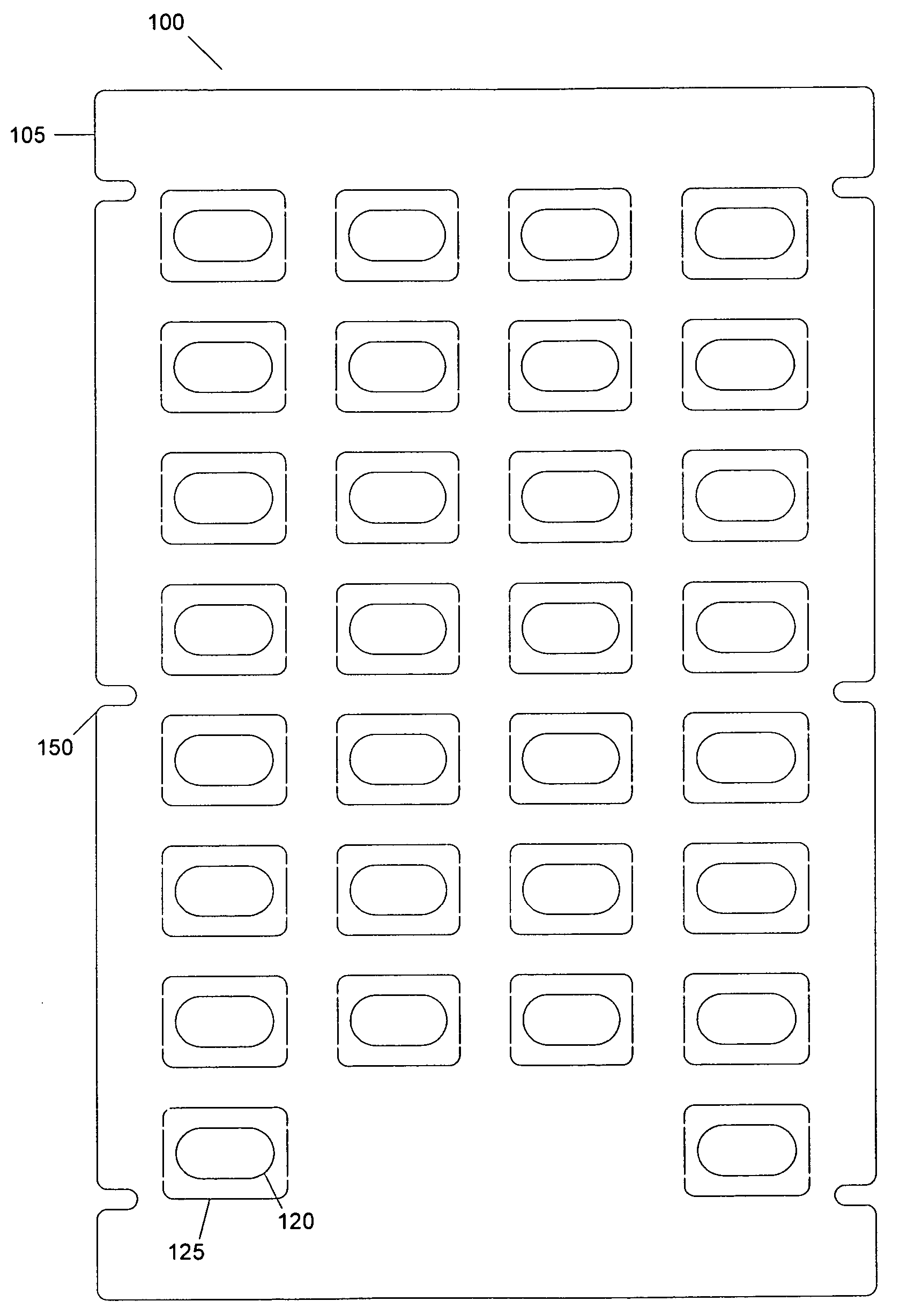
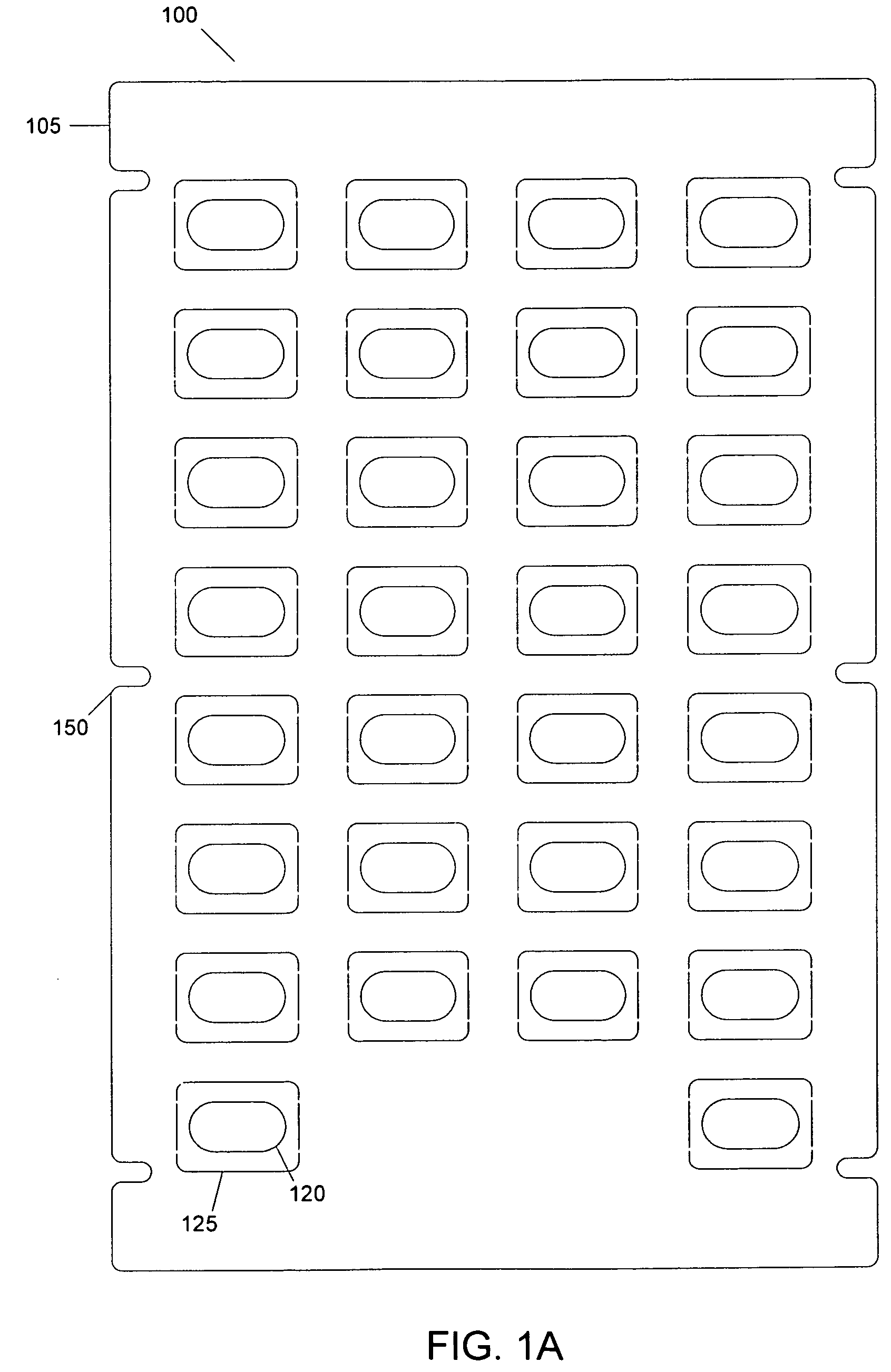
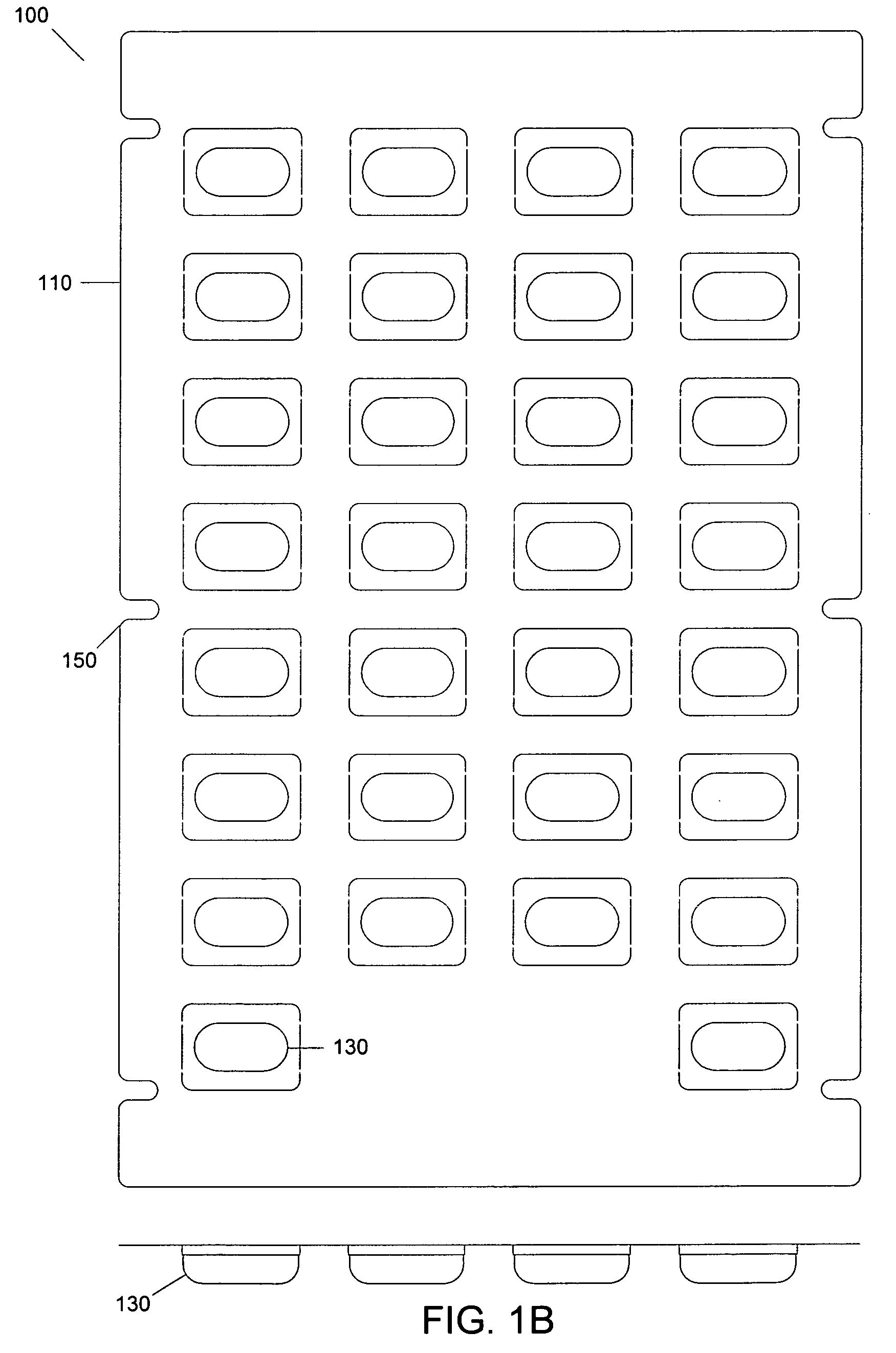
[0032]FIGS. 1A-1C depict exemplary layers of a blister pack according to an embodiment. As shown in FIG. 1, a blister pack 100 may include a first layer 105 (shown in FIG. 1A), a second layer 110 (shown in FIG. 1B) and a third layer 115 (shown in FIG. 1C). The first layer 105 of the blister pack 100 may include a bottom board. The bottom board may have one or more holes, such as 120. In an embodiment, a first area 125 may surround a hole 120. In an a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com