Vascular implant
a technology of vascular implants and sleeve, which is applied in the field of intravascular implants, can solve the problems of constricted ring member parts, sleeve gaps, and ring members, and achieve the effects of reducing the diameter of the lumen, reducing the ring diameter, and being easy to deploy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
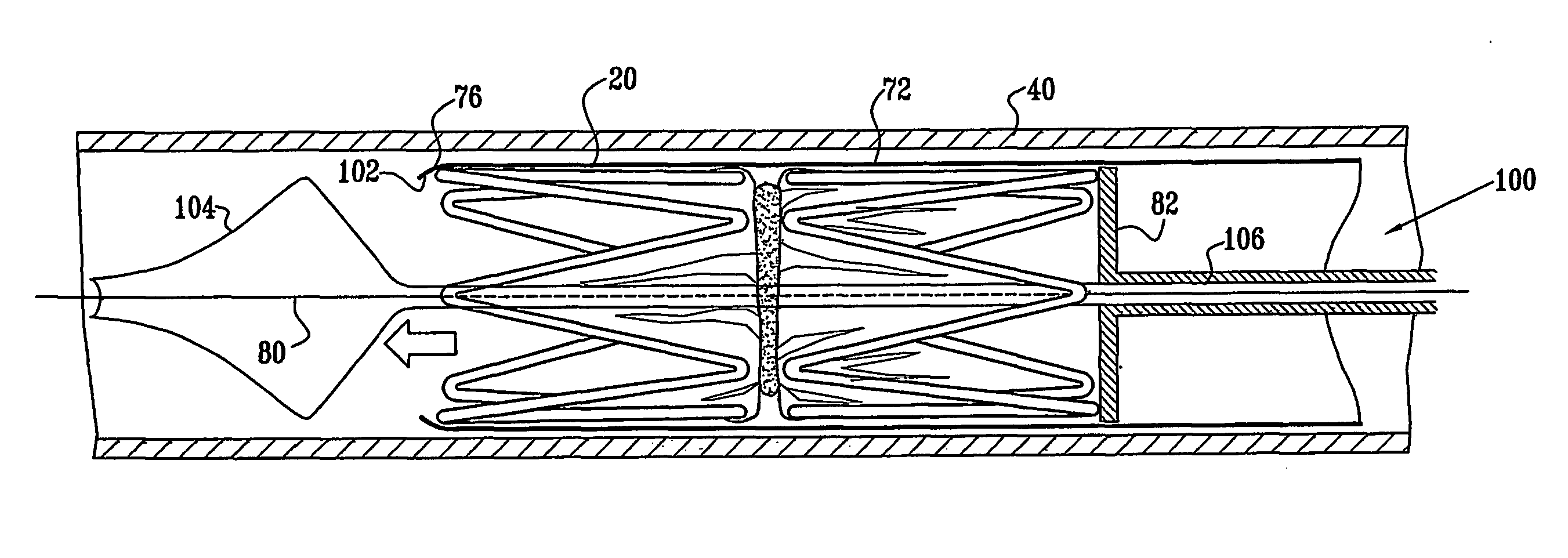
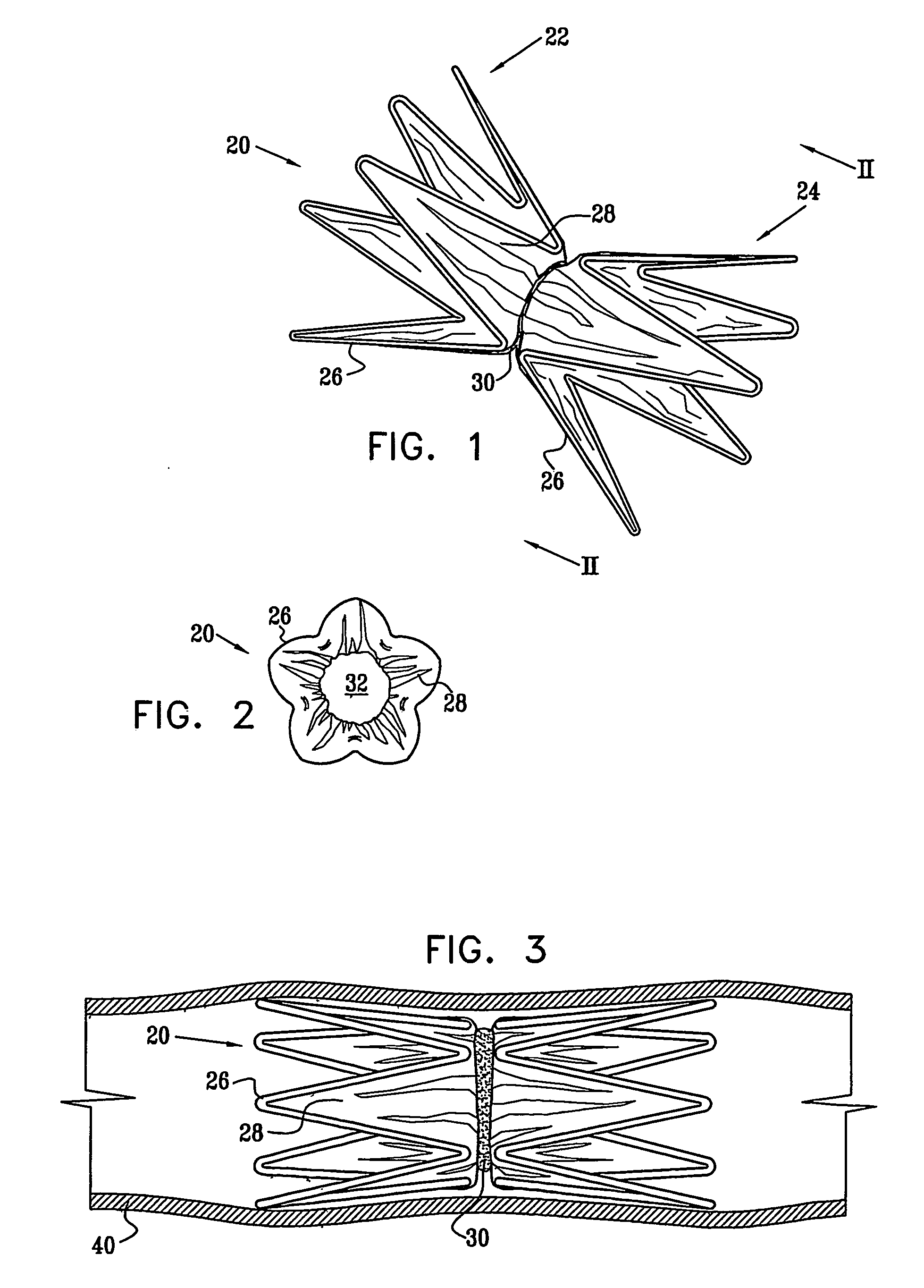
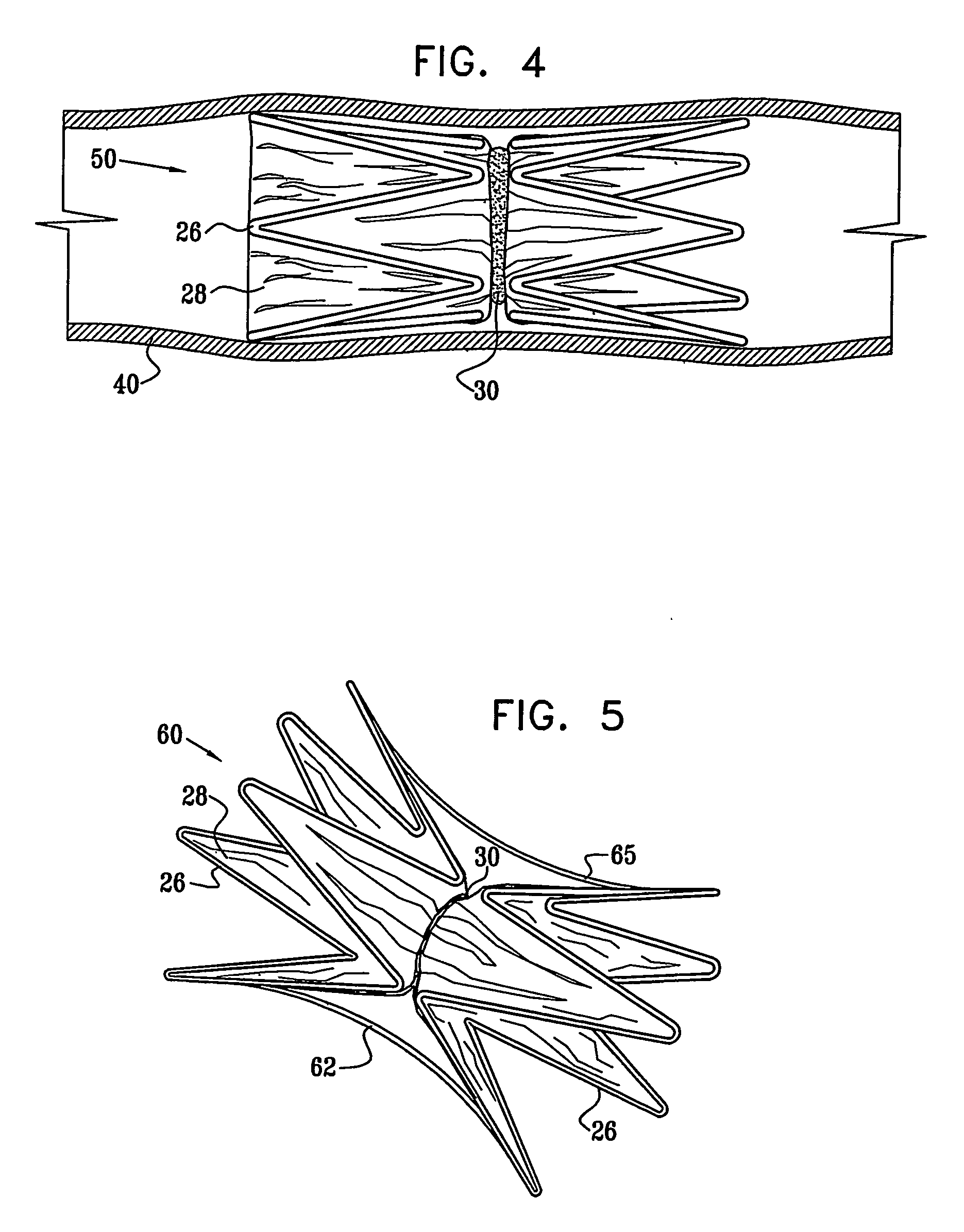
[0068] Reference is now made to FIGS. 1 and 2, which schematically illustrate a device 20 for implantation in a body passage, in accordance with an embodiment of the present invention. FIG. 1 is a pictorial illustration of the device, while FIG. 2 is a cross-sectional view taken along a line II-II in FIG. 1. Device 20 is adapted for use particularly in restricting blood flow through the coronary sinus, as described in the above-mentioned PCT Publication WO 01 / 72239. Alternatively, devices in accordance with the principles of the present invention may be implanted elsewhere in the vascular system, as well as in other body passages. For the sake of simplicity and clarity, however, and not limitation, embodiments of the present invention are described hereinbelow with reference to implantation of flow-constricting devices in blood vessels, such as the coronary sinus.
[0069] Device 20 comprises ring elements 22 and 24, each of which comprises a resilient framework 26. Each framework def...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com