Stent delivery system and method of manufacturing same
a delivery system and a technology of crocheted cords, applied in the field of medical stents, can solve the problems of difficult manufacturing of the aforementioned crocheted cord delivery system, difficult manufacturing of the delivery system, and inability to meet the needs of self-expandable stents with limited axial strength, etc., and achieves the effect of convenient use and easy manufacturing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
first embodiment
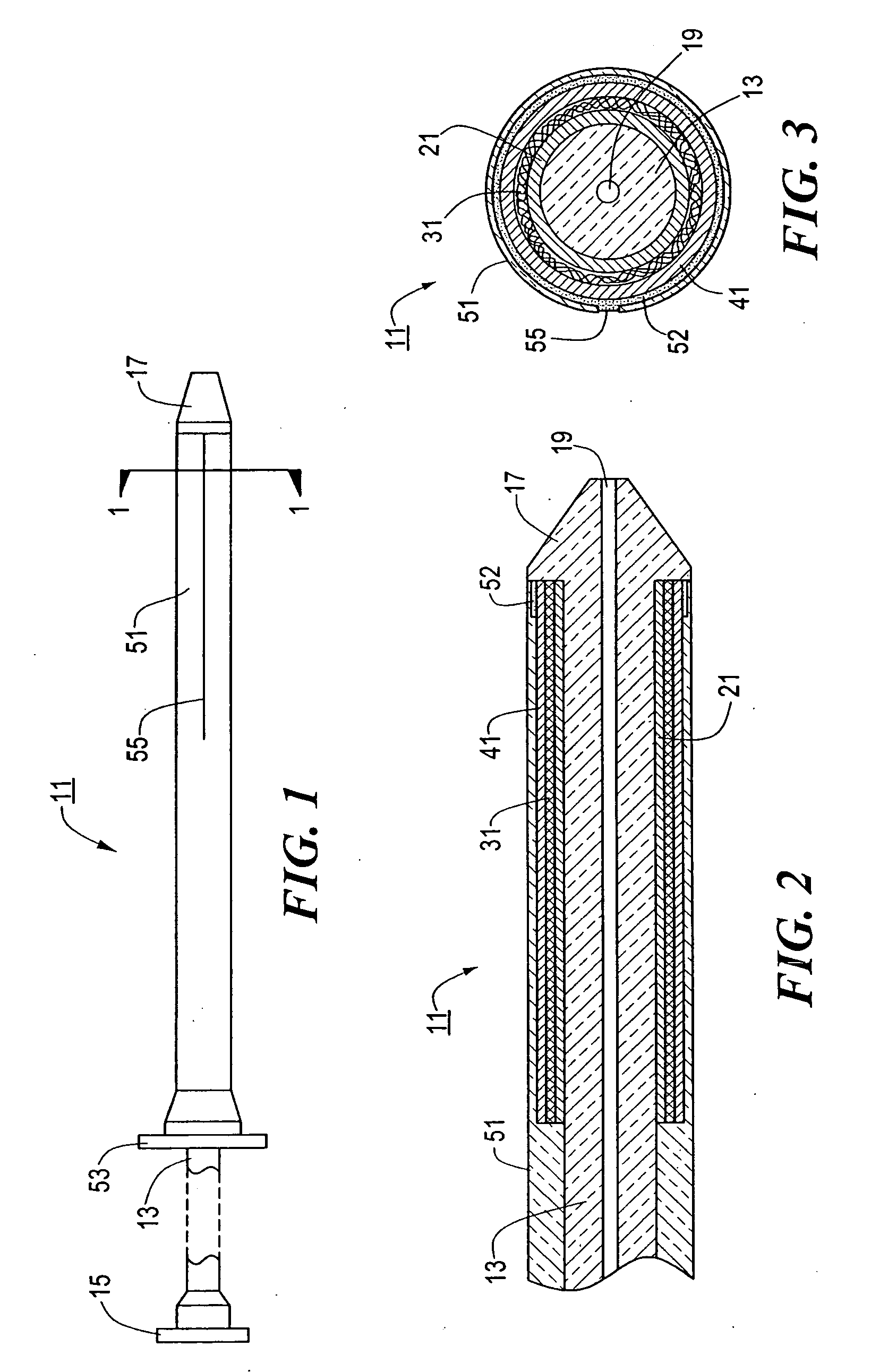
[0053] Referring now to FIGS. 1 through 3, there are shown various views of a stent delivery system constructed according to the teachings of the present invention, said stent delivery system being represented generally by reference numeral 11.
[0054] System 11 comprises a flexible, inner catheter 13. A handle 15 is disposed at the proximal end of catheter 13, and an enlarged tip 17 is disposed at the distal end of catheter 13. A lumen 19 extends longitudinally through catheter 13, lumen 19 being adapted to receive a guide wire for use in positioning system 11 at a desired position within a body lumen.
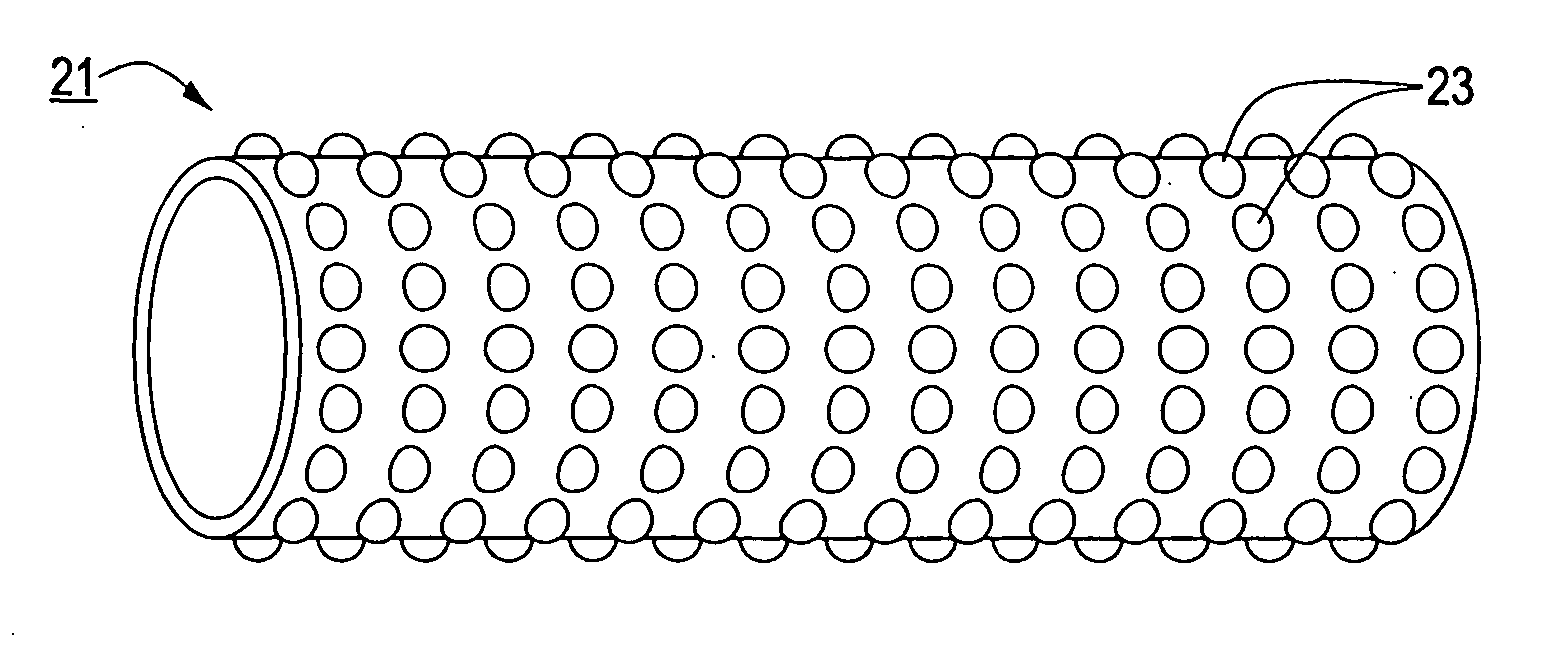
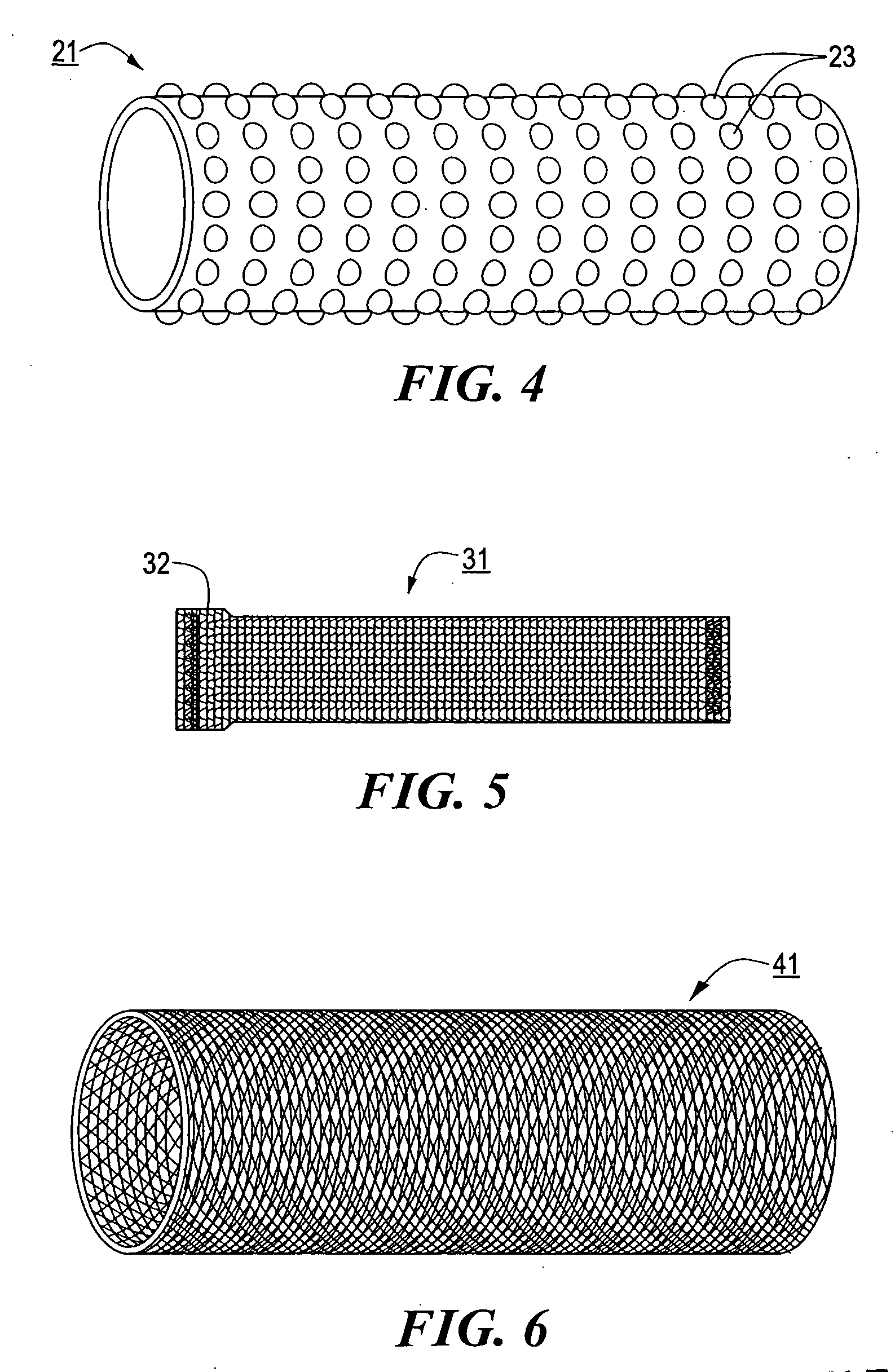
[0055] System 11 further comprises a stent engaging sleeve 21, sleeve 21 coaxially surrounding and secured (e.g., by a frictional fit) to that portion of catheter 13 proximally contiguous to tip 17. The primary purpose of sleeve 21 is to engage a stent mounted thereover in such a way as to prevent said stent, during deployment, from sliding proximally relative to catheter 13. To this e...
third embodiment
[0065] Referring now to FIGS. 10 and 11, there are shown longitudinal and transverse section views, respectively, of a stent delivery system constructed according to the teachings of the present invention, said stent delivery system being represented generally by reference numeral 101.
[0066] System 101 is similar in most respects to system 11, the principal difference between the two systems being that system 101 does not include sleeve 41 of system 11. Instead, system 101 relies on catheter 51 to function as the external restraint mechanism for keeping stent 31 in its compressed state until deployment. A lubricant (not shown) may be applied to the inside surface of catheter 51 to ensure that stent 31 does not axially compress, during deployment, as catheter 51 is moved proximally. As can readily be appreciated, catheter 51 of system 101 could be replaced with catheter 51′ and jacket 61.
[0067] System 101 is used in the same manner as system 11.
fourth embodiment
[0068] Referring now to FIGS. 12 and 13, there are shown longitudinal and transverse section views, respectively, of a stent delivery system constructed according to the teachings of the present invention, said stent delivery system being represented generally by reference numeral 201.
[0069] System 201 is similar in most respects to system 11, the principal difference between the two systems being that system 201 comprises, instead of sleeve 41, a stent restraining element in the form of a single helical coil 203 wrapped around stent 31. Coil 203 may be made of wire, thread, ribbon or like materials and may be wrapped around stent 31 either manually or with the use of an automated winding machine. The distal end of coil 203 is coupled to the distal end of catheter 51. One advantage to using coil 203, instead of sleeve 41, is that it is less complicated and less costly, particularly in terms of automated equipment, to apply a single, coiled constrainment element than it is to apply m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com