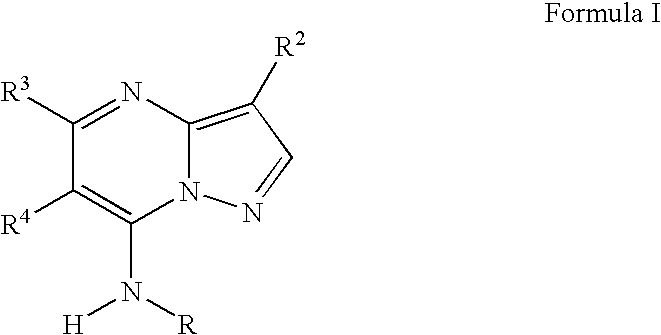
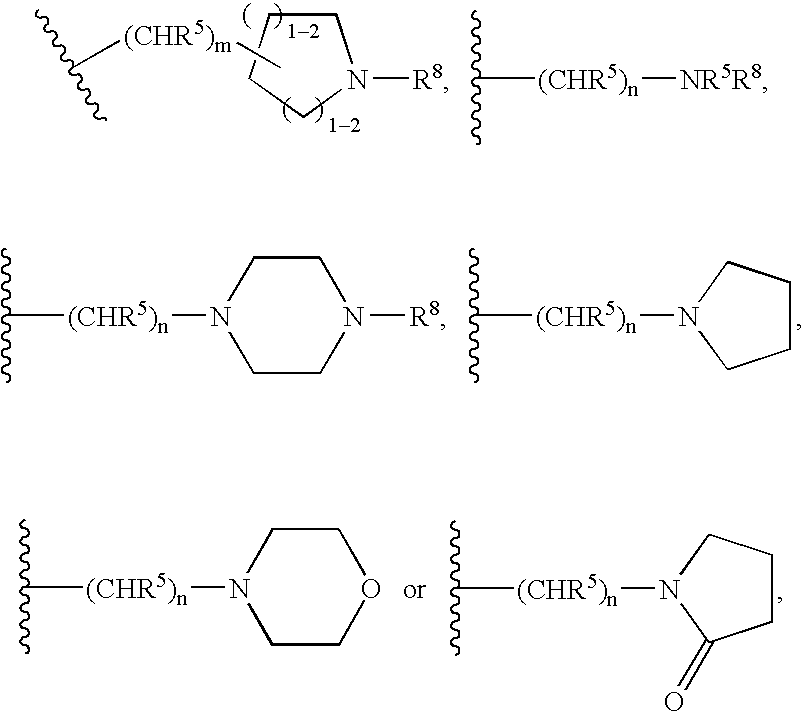
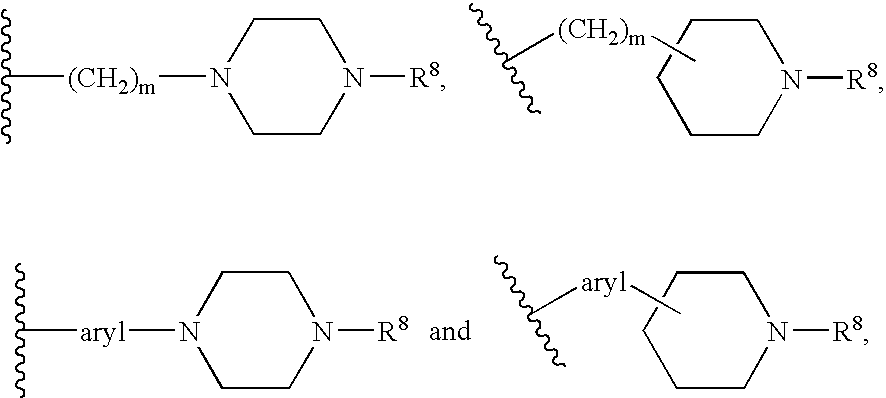
Methods for inhibiting protein kinases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
PREPARATIVE EXAMPLE 1
[0429]
[0430] SOCl2 (18.5 mL) was added slowly under N2 to a stirred mixture of the acid (50.0 g, 218 mmol) and pyridine (44.0 mL) in anhydrous CH2Cl2 (300 mL). The mixture was stirred at 25° C. for 20 min, then Meldrum's acid (35.0 g, 243 mmol) and DMAP (66.6 g, 546 mmol) were added and the mixture was stirred under N2 for 1 hr. Then Et2O (2 L) was added, the mixture was washed with 1 M HCl (3×500 mL), brine (500 mL), and the organic layer was dried over Na2SO4, filtered, and the solvent was evaporated. The residue was dissolved in MeOH (580 mL), and the mixture was refluxed for 4 hr. The solvent was evaporated and the residue was purified by column chromatography on silica gel with 10:1 CH2Cl2 / EtOAc as eluent. Pale yellow oil (26.5 g, 43%) was obtained.
example 2
PREPARATIVE EXAMPLE 2
[0431]
[0432] A mixture of the beta-ketoester from Preparative Example 1 (20.0 g, 70.1 mmol) and 3-aminopyrazole (5.40 g, 65.0 mmol) in anhydrous toluene (60 mL) was stirred and refluxed under N2 for 24 hr. The solvent was evaporated and the residue was purified by column chromatography on silica gel with 20:1 CH2Cl2 / MeOH as eluent. White solid (15.0 g, 73%) was obtained. LC-MS: 319 [M+H].
example 3-4
PREPARATIVE EXAMPLE 3-4
[0433] By essentially same procedure set forth in Preparative Example 2, combining 3-aminopyrazole with the corresponding beta-ketoesters, compounds given in Column 1 of Table 1A were prepared.
TABLE 1AEx.Column 1Data3LCMS: MH+ = 2364
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com