Passively temperature-regulated shipping container suitable for biological, pharmaceutical materials or food products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
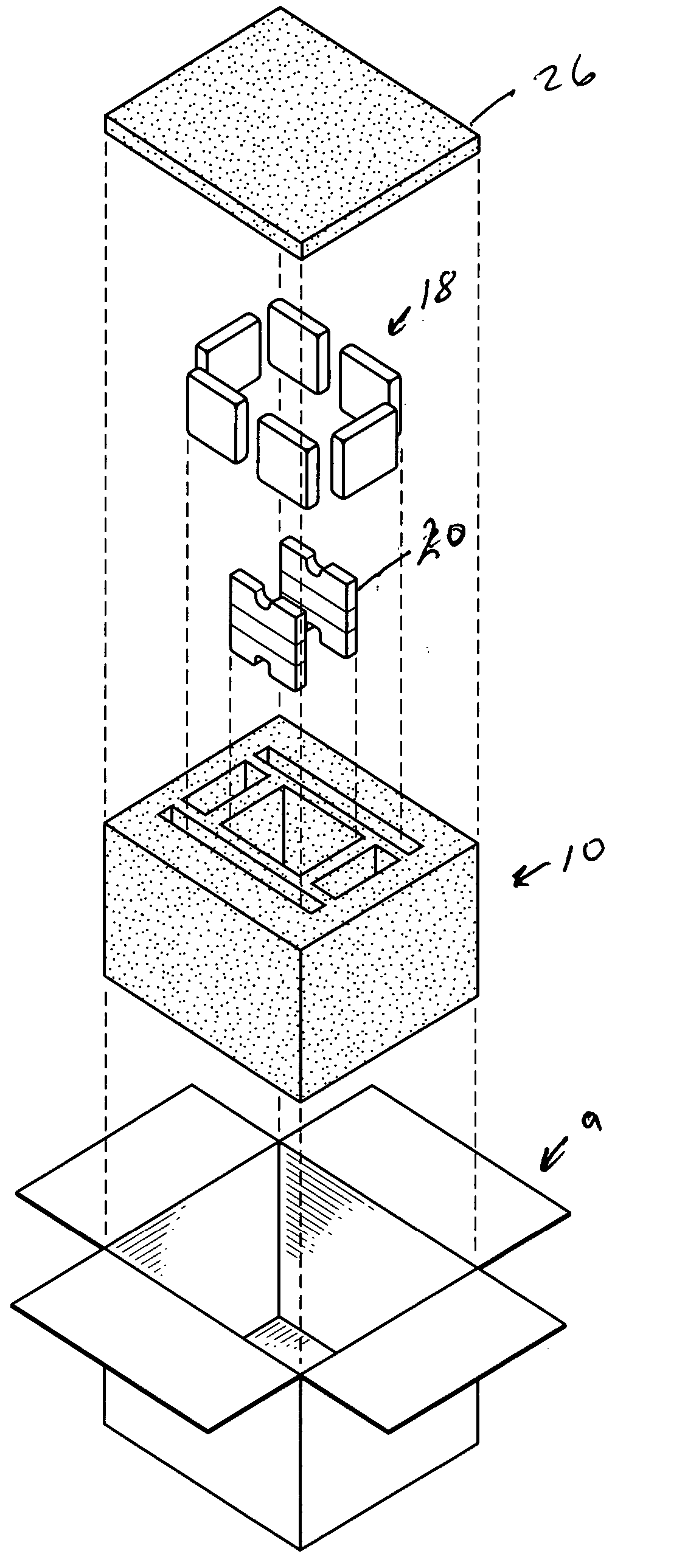
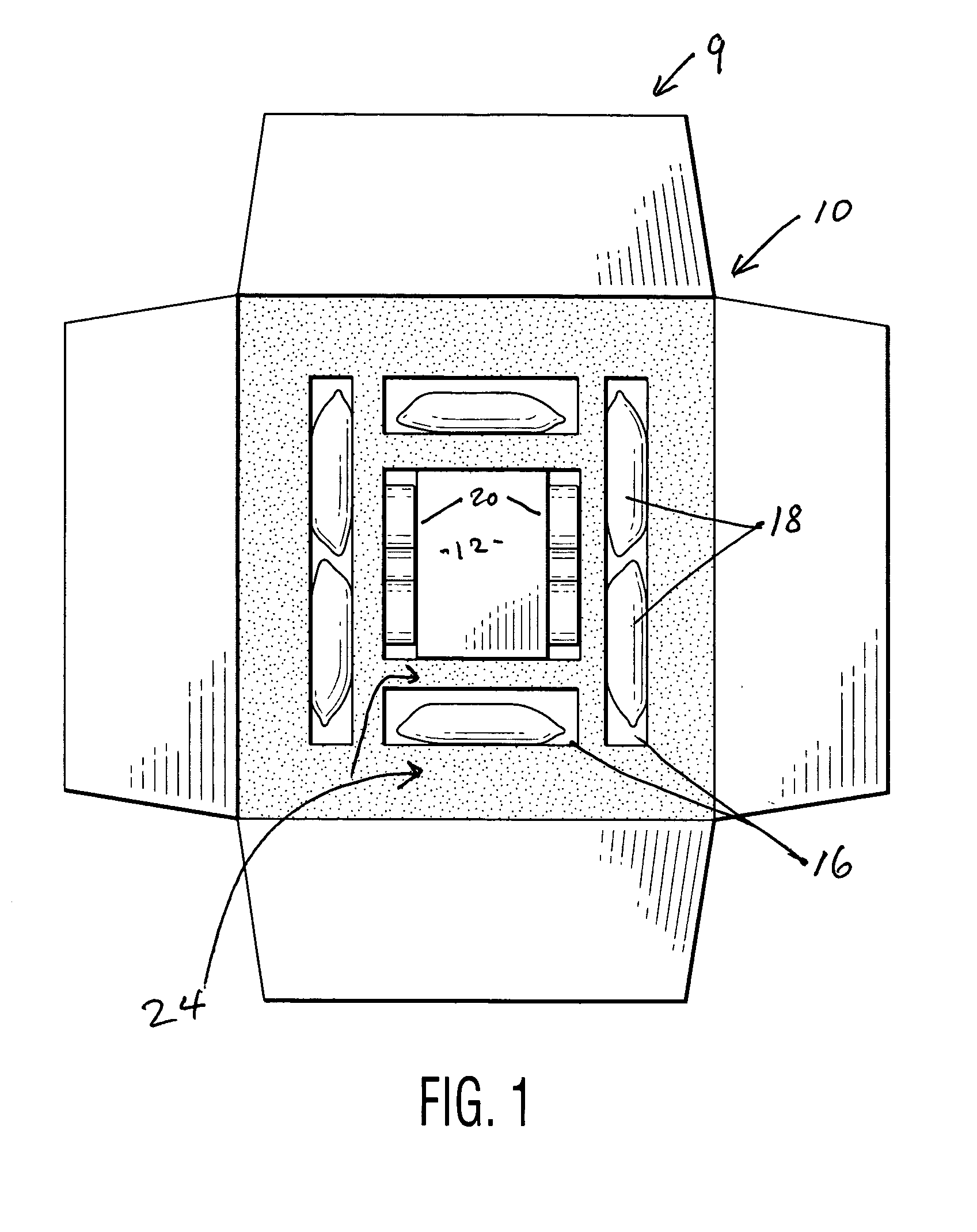
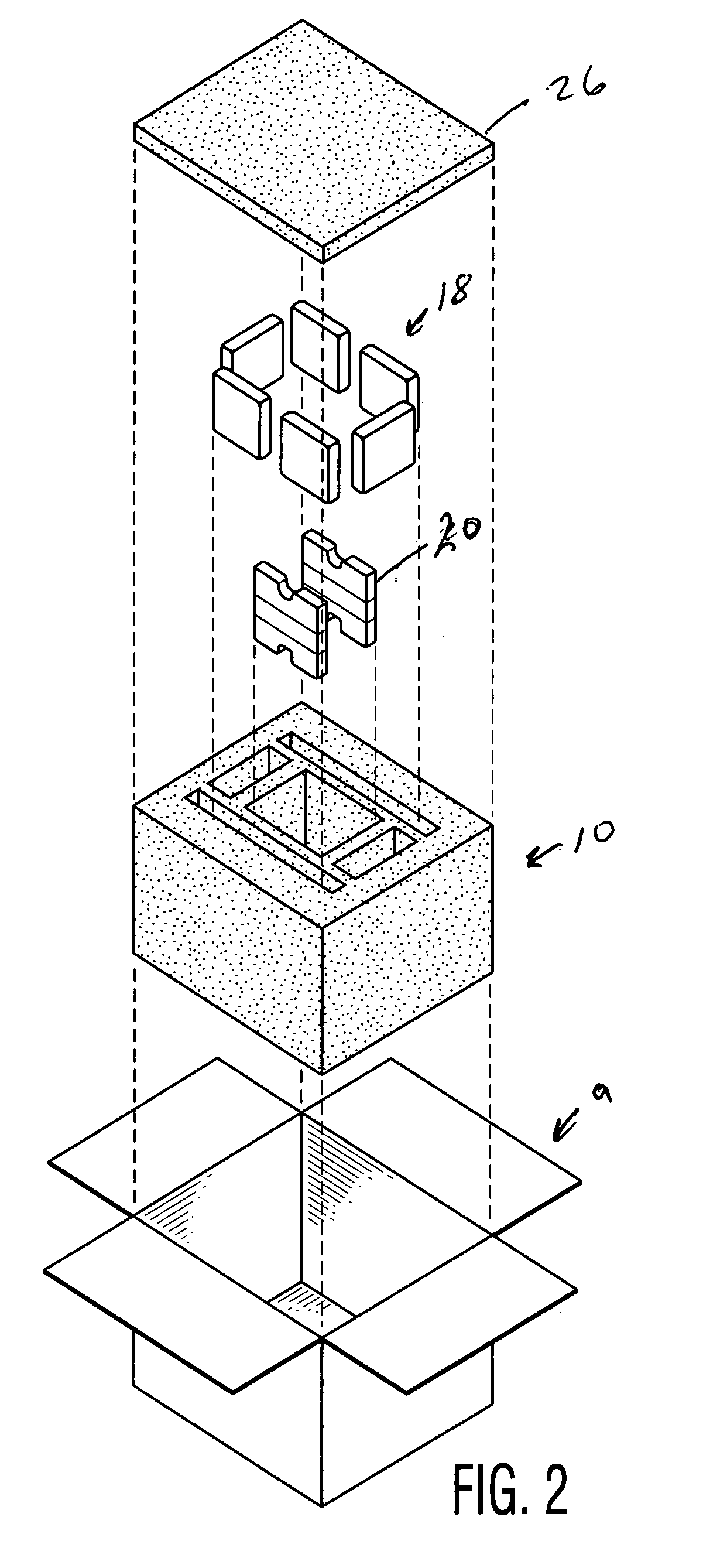
[0010]FIG. 1 depicts a shipping container 10, inside a container 9, and having a payload chamber 12, and a series of first chambers 16. Ice (depicted in the form of gel packs 18) is placed in the first chambers 16. Individual containers 20 containing a first phase change material is placed in the payload chamber 12, which also houses a payload, preferably pharmaceuticals, food or a biological material. Two containers 20 are shown, but more may be used, with four (so that the payload is completely surrounded) being preferred. Suitable phase change materials include those that change solid to liquid in the range of 2° to 8° C. for example, decanol, and other materials including many set forth in US Patent No. 5,647,226: “Phase change apparatus for animal parts, human body parts, body fluids and culture” (incorporated by reference). It is also possible to use phase change materials outside this range for particular purposes, for example, dodecanol. Instead of ice it is possible to use ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com