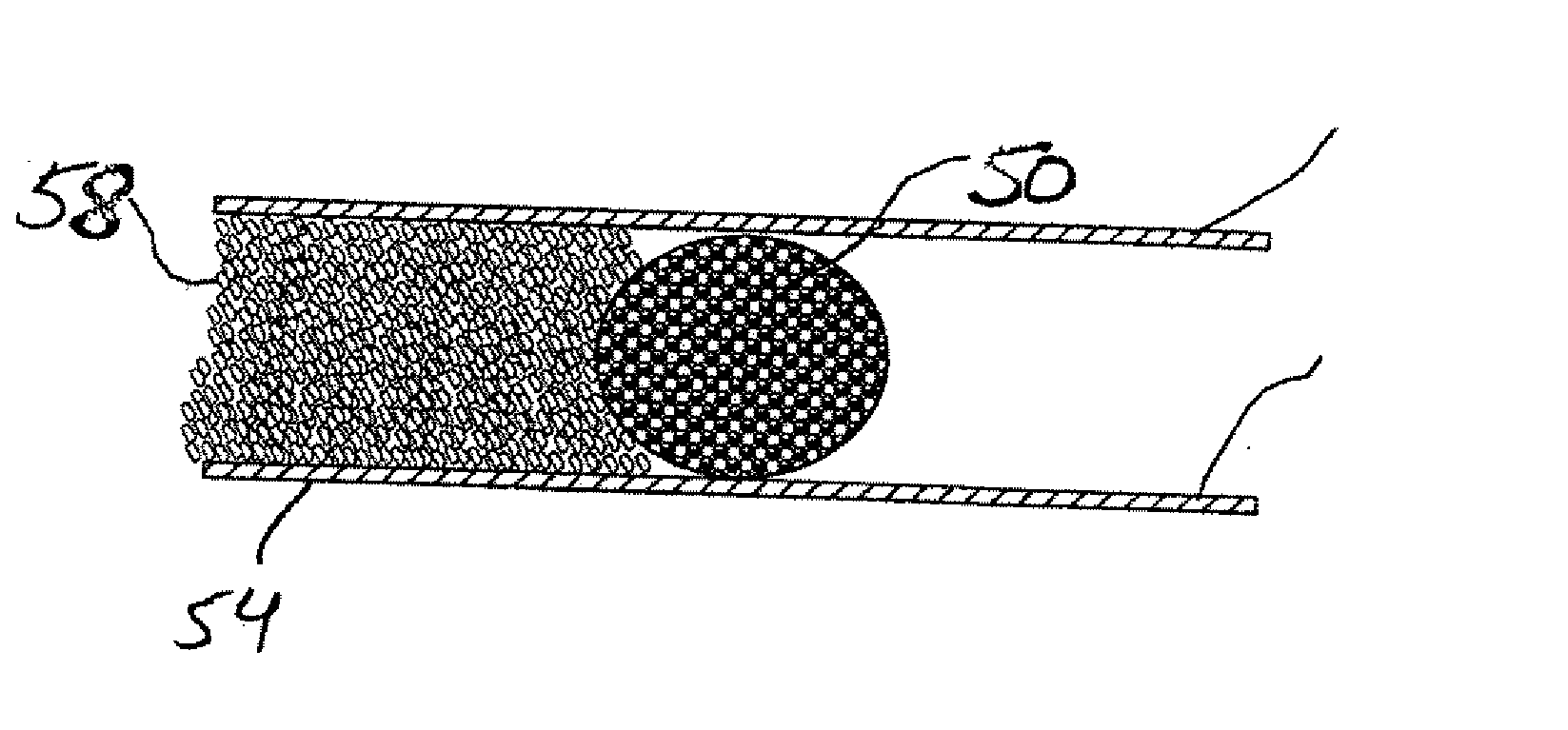
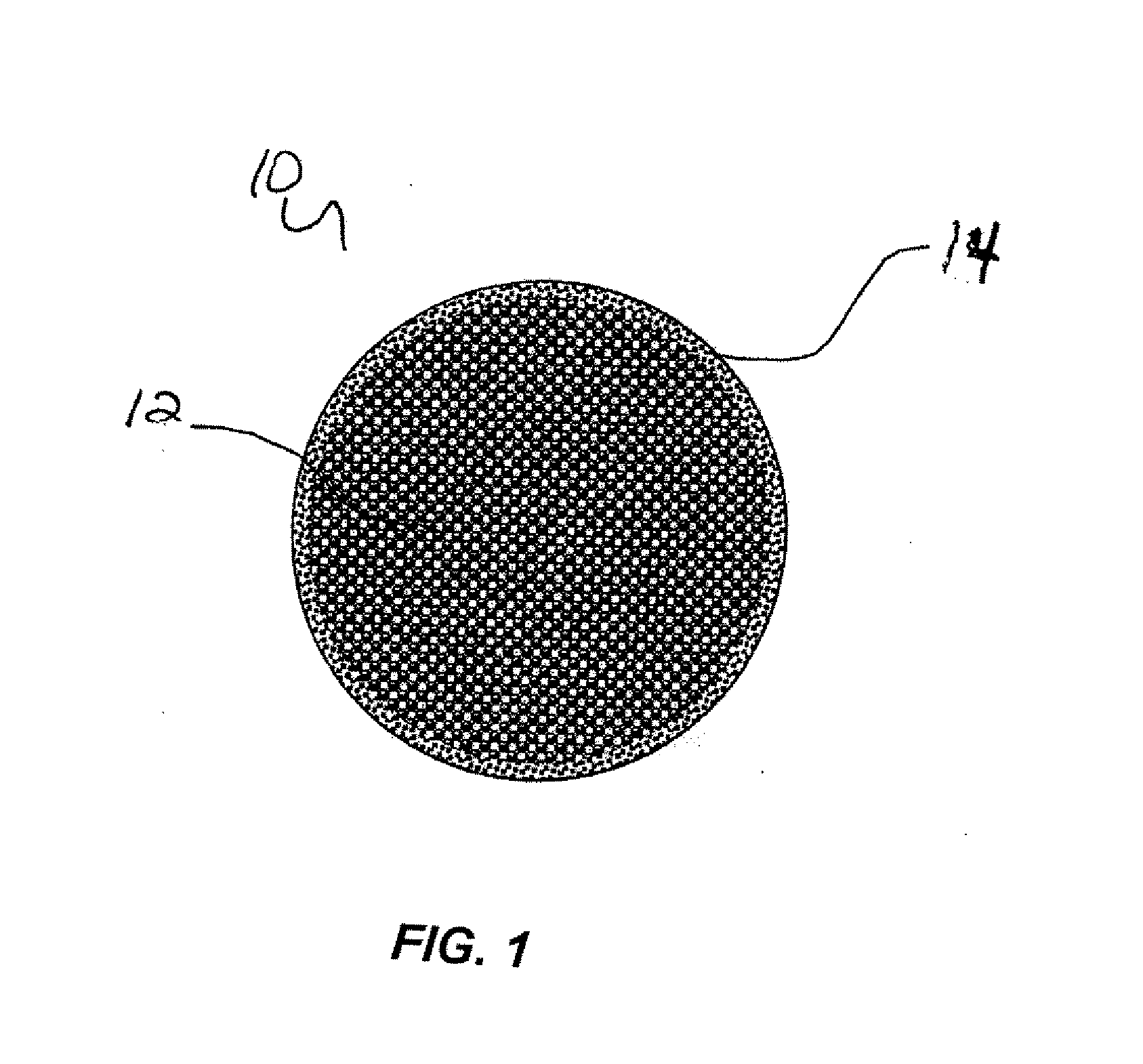
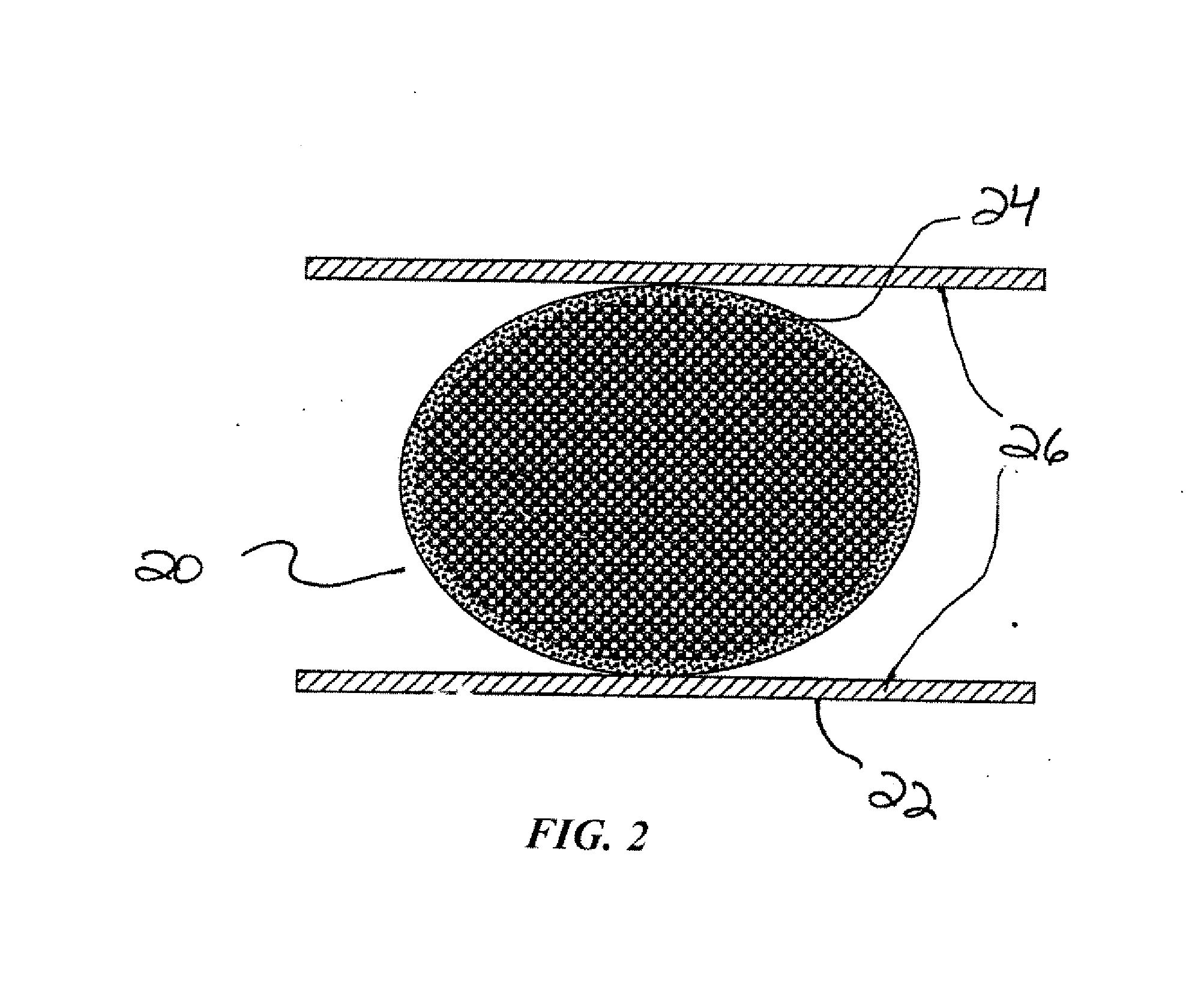
Compressible intravascular embolization particles and related methods and delivery systems
a technology of intravascular embolization and compression particles, which is applied in the field of compression particles, can solve the problems of inadvertent downstream embolization, poor positioning control, and perforation of blood vessels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0070] A crosslinked PVA embolization particle was prepared in the following manner. A mixture of 26.2 grams of PVA and 98.9 grams of deionized water was rapidly heated to 100° C. and held for 12 minutes. Subsequently, 39.9 grams of the resulting PVA solution was transferred for reaction purposes into a reaction kettle (a glass beaker) and set aside and allowed to cool. Separately, a mixture of 15 grams of rice starch and 135 grams of deionized water was heated to 80° C. and then 9.5 grams of the material was added to the PVA solution and thoroughly mixed. To this resulting mixture was added 3.6 grams of concentrated hydrochloric acid and 6.4 grams of about 37% formaldehyde (formalin solution) to form the reaction solution.
[0071] The reaction solution was then placed in a 2 liter glass reaction kettle and mixed at about 7000 rpm with a high-speed mixer having a high-speed air motor and using a mixing blade to whip air into the mixture until the foam stopped expanding and the result...
example 2
[0074] The same procedure performed in Example 1 was repeated, but the formaldehyde additive was reduced to 5.2 grams. It was believed that the reduction in formaldehyde would change the pot-life of the reaction solution, wherein “pot-life” is intended to mean the useful life of the mixture (after some period of time, the mixture ages to the point that it cannot be used to create a particle of the present invention). However, no significant change in pot-life was observed.
example 3
[0075] The same procedure performed in Example 1 was repeated, but the hydrochloric acid additive was reduced to 5.0 grams. The resulting sponges had both an increased firmness and increased resilience in comparison to the sponges produced in Example 1, wherein firmness is a qualitative measure of the compressibility of the particle. Further, the pot-life of the reaction material was decreased by about 30 seconds.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com