Pharmaceutical compositions
a technology of pharmaceutical compositions and compositions, applied in the direction of aerosol delivery, immunological disorders, metabolism disorders, etc., can solve the problem of fast uptak
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0112] A composition comprising delta-9-tetrahydrocannabinol (delta-9-THC) with HFC134a suitable for use in a device as described above can be formulated from the following ingredients:
Componentpercent w / wg / canDelta-9-THC0.70.099Ethanol 96% BP13.21.866Peppermint oil1.40.205HFC-134a84.712.02Total10014.19
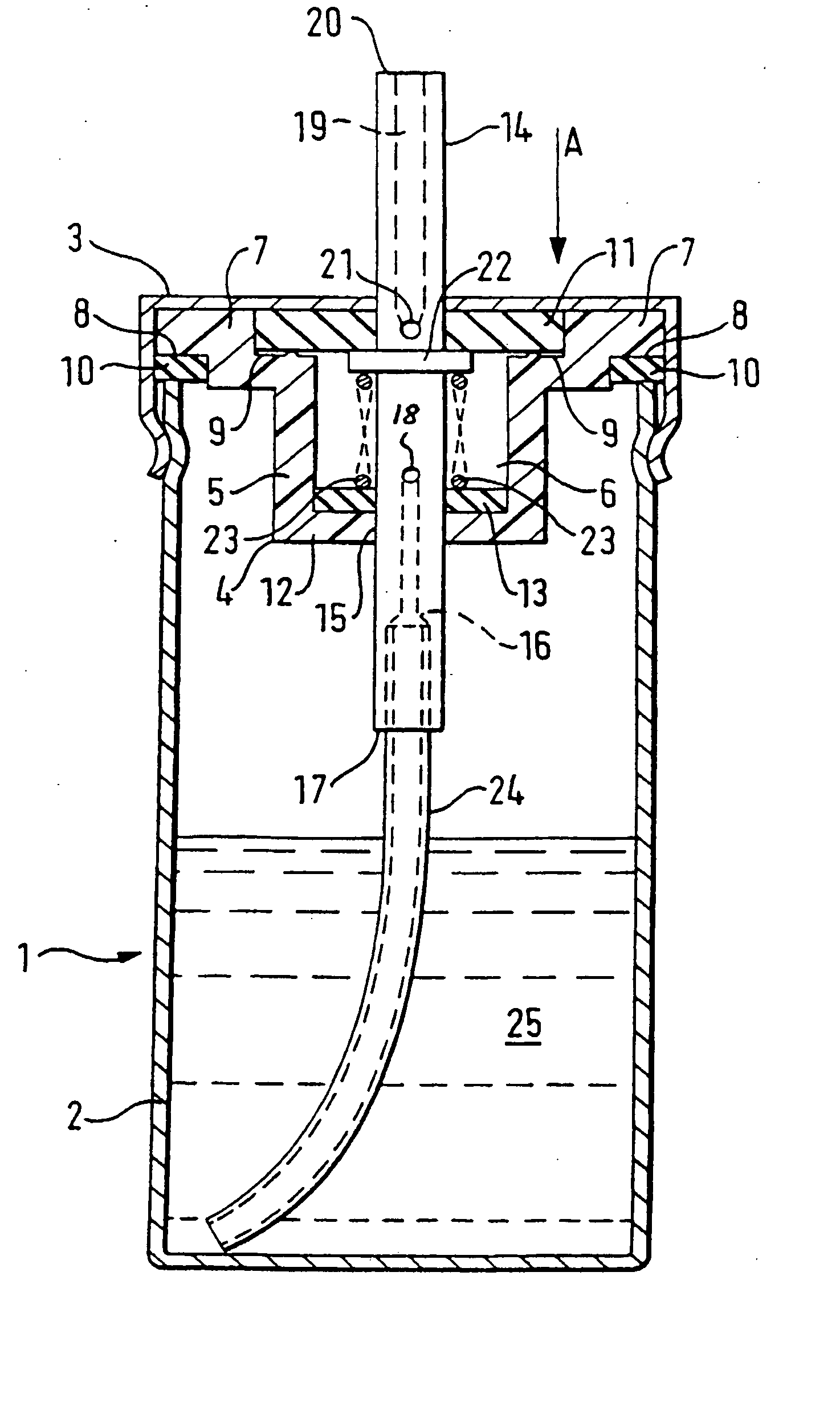
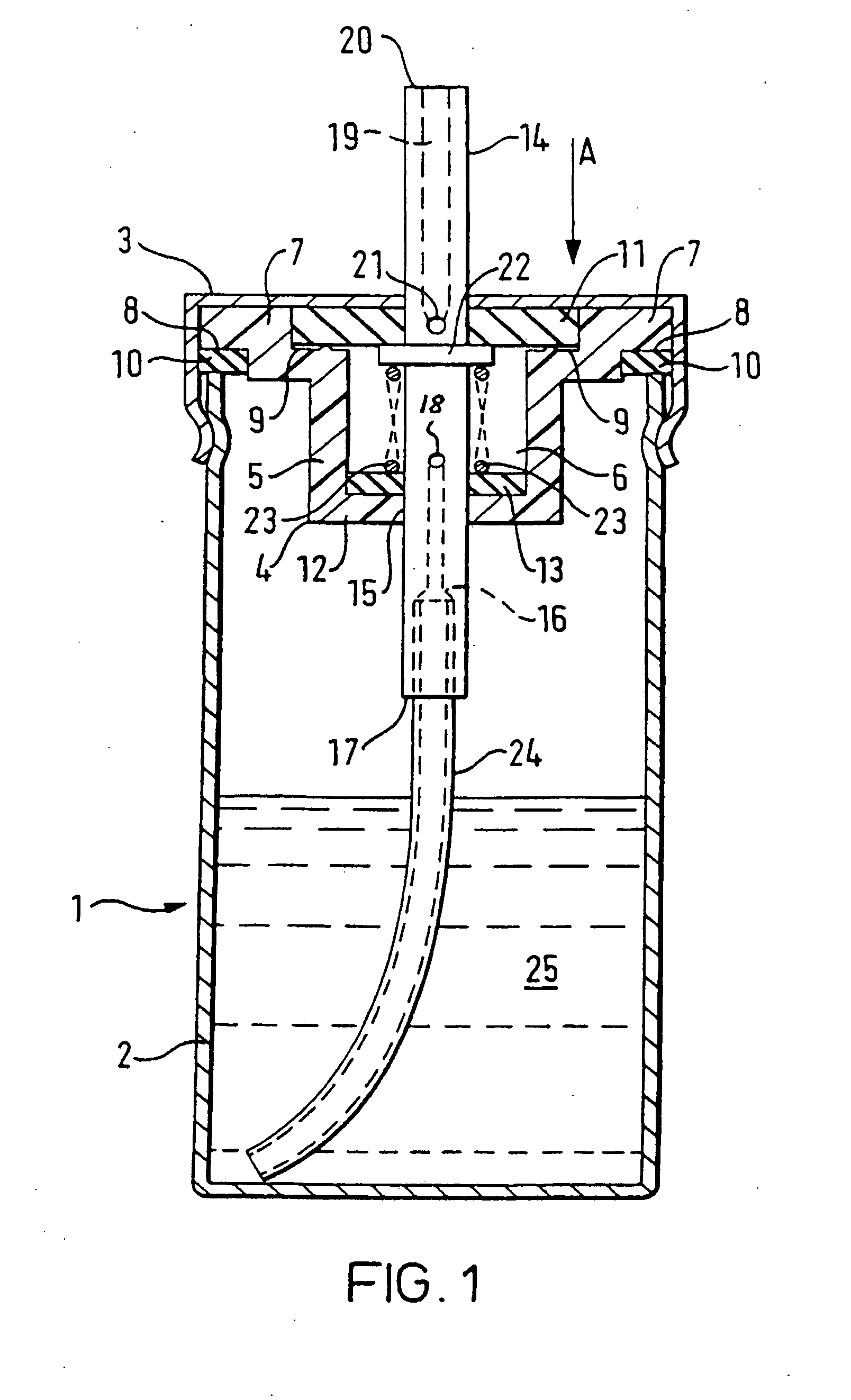
[0113] The peppermint oil is added to the delta-9-THC / ethanol solution and mixed thoroughly. 2.17 g of the resulting solution is then placed in the canister 2 and the valve assembly, comprising the valve body moulding 4, first sealing washer 11, second sealing washer 13, spring 22, tube 23, and annular seal 10 are then sealed onto the canister 2 as shown in FIG. 1 by the cap 3. The propellant is then added to the canister by being forced through the valve core 14 at great pressure, and the complete device is then checked for leaks.
example 2
[0114] A second composition comprising delta-9-THC with HFC-134a suitable for use in a device as described above can be formulated from the following ingredients:
Componentpercent w / wg / canDelta-9-THC0.1640.010Ethanol 96% BP4.9920.305HFC-134a94.8445.795Total1006.11
[0115] The delta-9-THC is dissolved in the ethanol in the proportions set out above and 0.315 g of the resulting solution is then placed in a canister 2 and a valve assembly, comprising a valve body moulding 4, first sealing washer 11, second sealing washer 13, spring 22, tube 23, and annular seal 10, is then sealed onto the canister 2 by crimping as shown in FIG. 1 by the cap 3. The propellant (HFC-134a) is then added to the canister, by being forced through the valve ore 14 at great pressure, and the complete device is then checked for leaks. After the propellant entered the canister it dissolves the remaining portions of the composition.
example 3
[0116] A third composition comprising delta-9-THC and suitable for use in a device as described above can be formulated from the following ingredients:
Componentpercent w / wg / canDelta-9-THC0.1640.010Ethanol 96% BP7.50.458HFC-134a92.3365.641Total1006.11
[0117] The delta-9-THC is dissolved in the ethanol in the proportions set out above and 0.315 g of the resulting solution is then placed in a canister 2. A valve assembly (as described in Example 2) is then sealed onto the canister 2 by crimping and the HFC-134a propellant is then added to the canister, by being forced through the valve core 14 at great pressure, and the complete device is then checked for leaks. After the propellant entered the canister it dissolves the remaining portions of the composition.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com