Reactive Working Material for Use in Hydrogen Production by Decompostion of Water
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
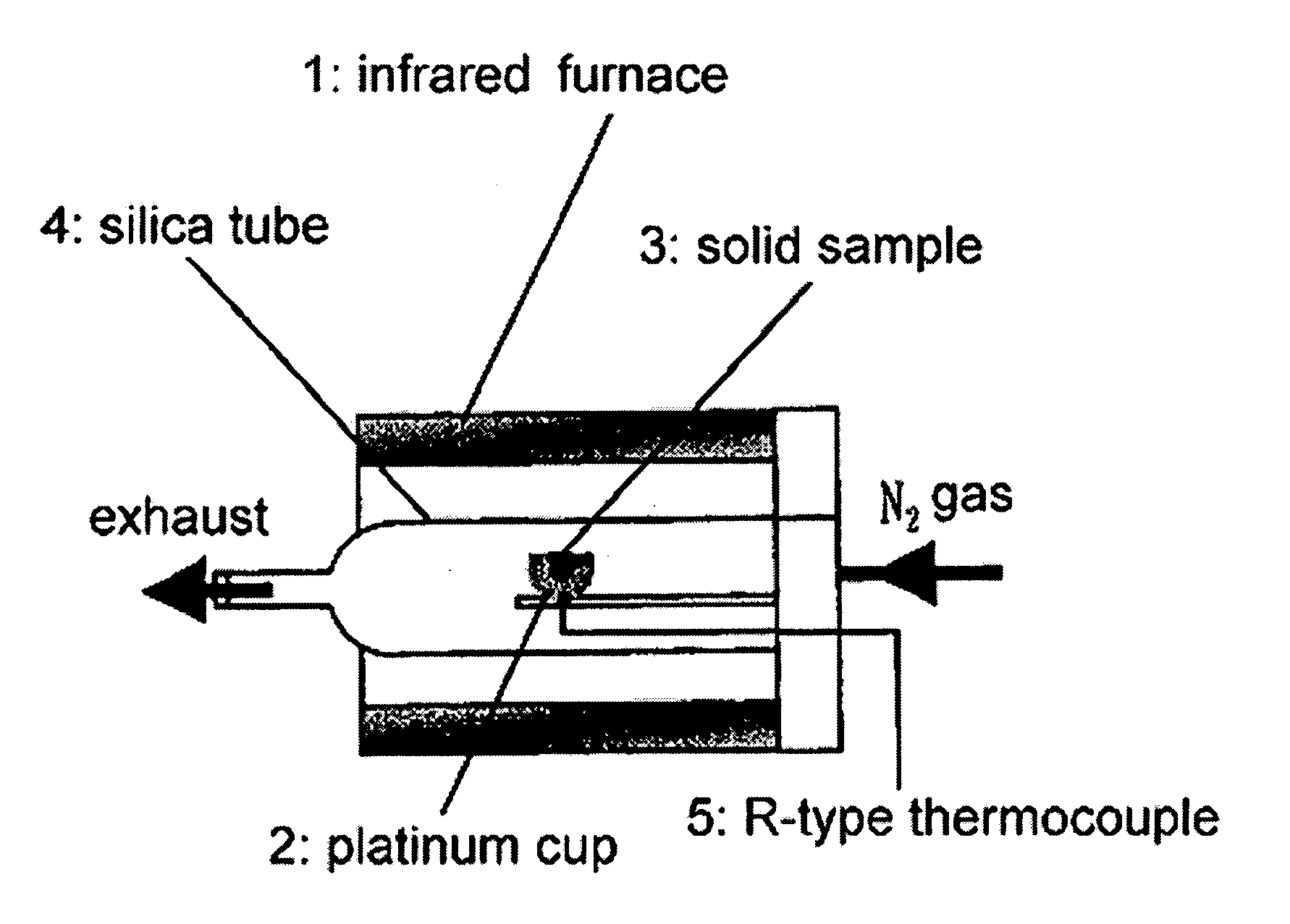
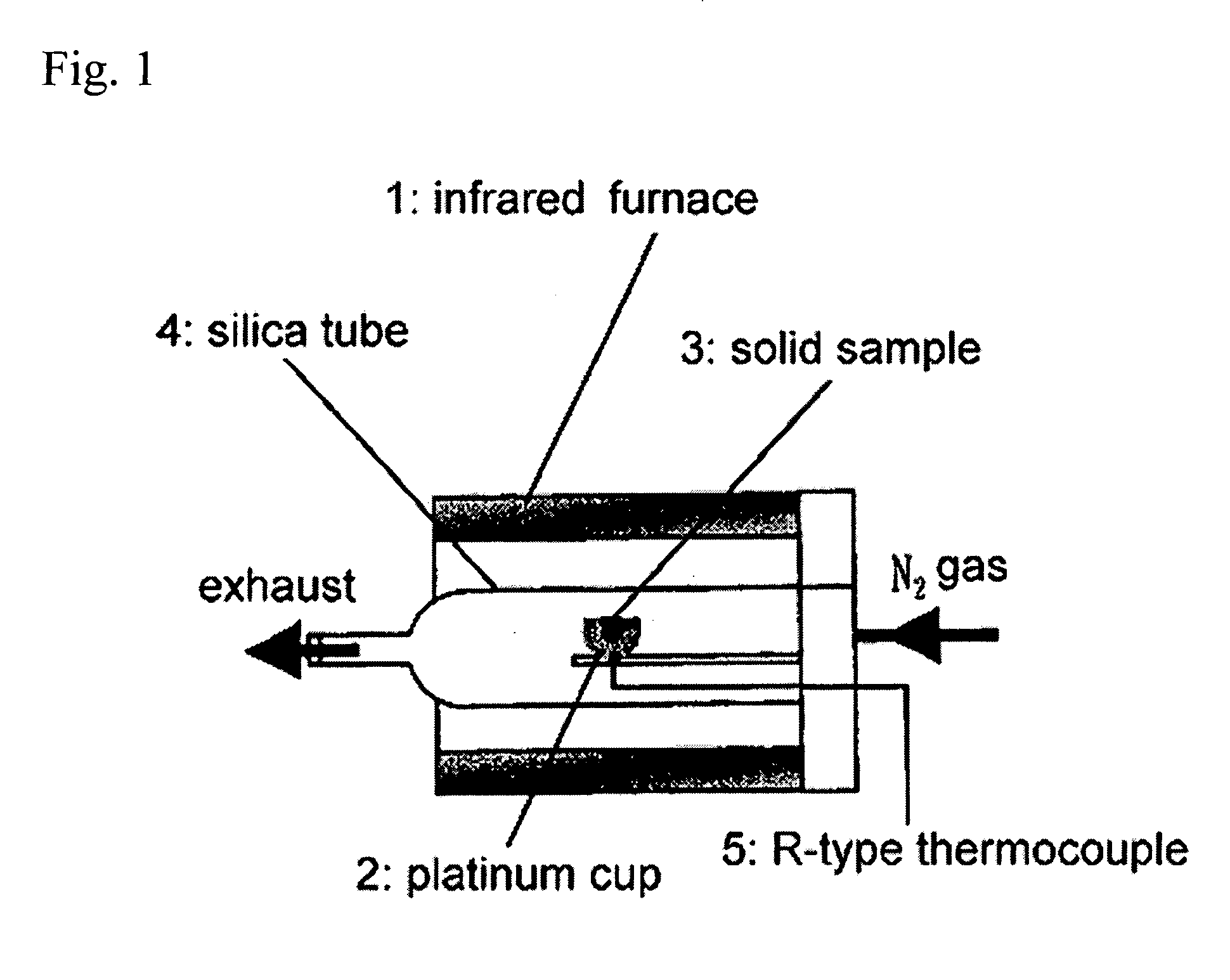
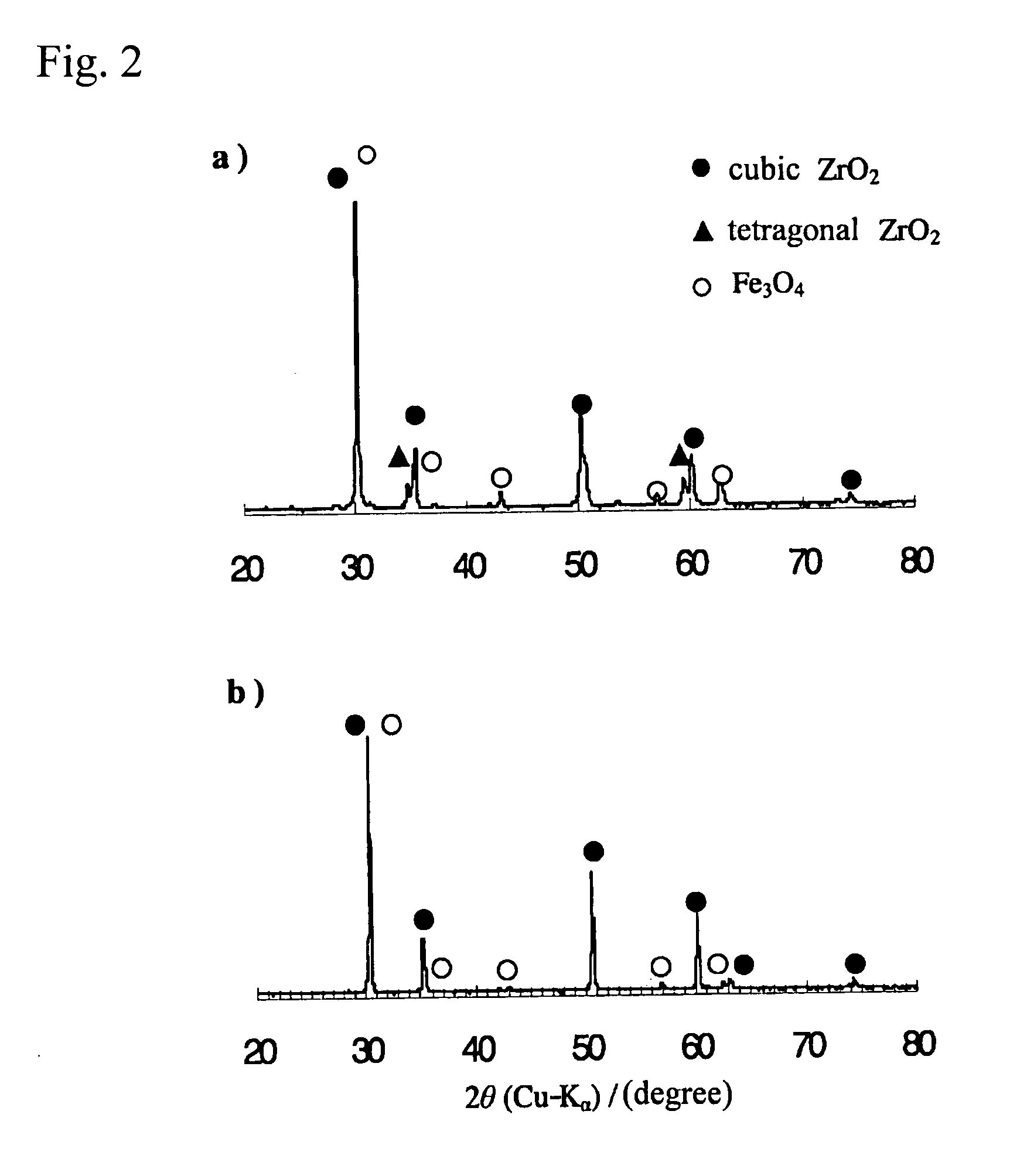
[0036] An yttria partially-stabilized zirconia (YSZ) represented by (ZrO2)0.97 (Y2O3)0.03 and a calcia partially-stabilized zirconia (CSZ) represented by (ZrO2)0.97 (CaO)0.03 (produced by Kojundo Chemical Lab. Co. Ltd.) were used as a support of a ferrite. Each of the YSZ and CSZ has a particle size of 1 μm or less. The YSZ primarily comprises a cubic crystal phase and slightly has a tetragonal crystal phase. The YSZ has a BET (Brunauer-Emmett-Teller) surface area of 7.7 m2g−1. The CSZ primarily comprises a cubic crystal phase and partially has a monoclinic crystal phase. As Comparative Example, a conventional monoclinic zirconia (BET surface area: 12.6 m2g−1) was used as a support.
[0037] A cubic zirconia-supported ferrite as Inventive Example and a monoclinic zirconia-supported ferrite as Comparative Example were prepared through the following process.
[0038] The zirconia particles were suspended in distilled water after removing oxygen and CO2 therefrom, and N2 was supplied there...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com