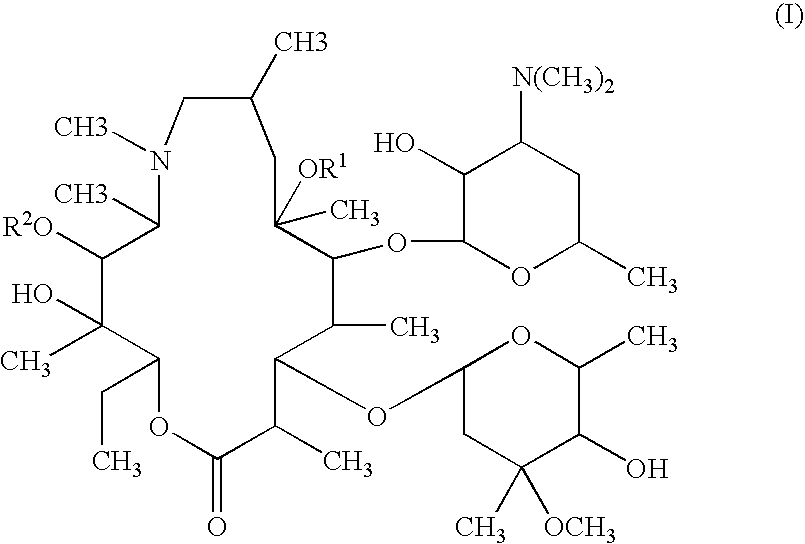
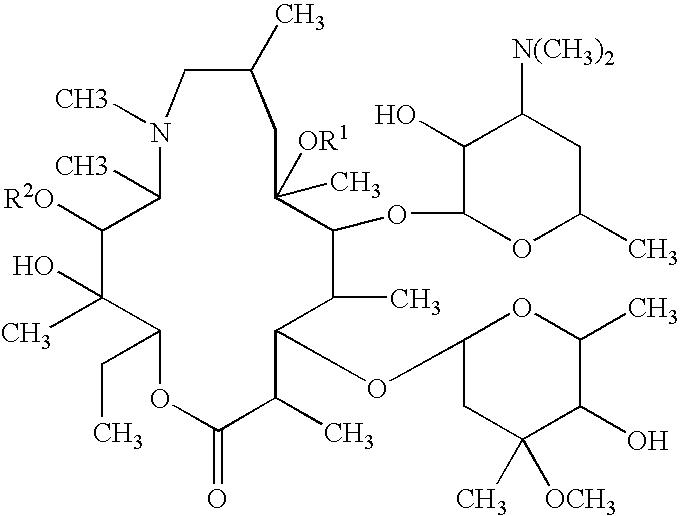
Topical treatment or prevention of ocular infections
a technology for ocular infections and treatment, applied in the direction of antibacterial agents, aerosol delivery, prosthesis, etc., can solve the problems of eyelids, conjunctiva, cornea, eyelid infections, eyelid infections, etc., and achieve the effect of preventing ocular infections, challenging and/or problematic treatment of ocular infections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 1-2
[0052]Hydroxypropylmethyl cellulose, sodium chloride, edetate sodium (EDTA), BAK and surfactant are dissolved in a beaker containing approximately ⅓ of the final weight of water and stirred for 10 minutes with an overhead stirred. The azithromycin is added and stirred to disperse for 30 minutes. The solution is sterilized by autoclaving at 121° C. for 20 minutes. Alternately, the azithromycin may be dry heat sterilized and added by aseptic powder addition after sterilization. Mannitol, Poloxamer 407, and boric acid are dissolved separately in approximately ½ of the final weight of water and added by sterile filtration (0.22 um filter) and stirred for 10 minutes to form a mixture. The mixture is adjusted to desired pH with 10N sodium hydroxide while stirring, brought to a final weight with water by sterile filtration and aseptically filled into multi-dose containers.
examples 3-6
[0053]Noveon AA-1 is slowly dispersed into a beaker containing approximately ⅓ of the final weight of water and stirred for 1.5 hrs. with an overhead stirrer. Noveon AA-1 is an acrylic acid polymer available from B. F. Goodrich. Edetate sodium (EDTA), BAK, sodium chloride, and surfactant are then added to the polymer solution and stirred for 10 minutes after each addition. The polymer suspension is at a pH of about 3.0-3.5. The azithromycin is added and stirred to disperse for 30 minutes. The mixture is sterilized by autoclaving at 121° C., for 20 minutes. Alternately, the azithromycin may be dry heat sterilized and added by aseptic powder addition after sterilization. Mannitol, and boric acid, or sodium perborate, Dequest, mannitol, and boric acid are dissolved separately in approximately ½ of the final weight of water, added to the polymer mixture by sterile filtration (0.22 um filter) and stirred for 10 minutes. The mixture is adjusted to the desired pH with ION sodium hydroxide ...
example 7
[0054]Noveon AA-1 is slowly dispersed into a beaked containing approximately ½ of the final weight of water and stirred for 1.5 hrs. With overhead stirrer Noveon AA-1 is an acrylic acid polymer available from B. F. Goodrich. Edetate sodium (EDTA), Poloxamer 407, and sodium chloride are then added to the polymer suspension and stirred for 10 minutes. The polymer suspension is at a pH of about 3.0-3.5. The azithromycin is added and stirred to disperse for 30 minutes. The mixture is sterilized by autoclaving at 121° C. for 20 minutes. Alternately, the azithromycin may be dry heat sterilized and added by aseptic powder addition after sterilization. Mannitol is dissolved in 1 / 10 of the final weight of water and sterile filtered (0.22 um filter) in to the polymer suspension and stirred for 10 minutes. The mixture is adjusted to desired pH with ION sodium hydroxide while stirring, brought to final weight with water by sterile filtration and aseptically filled into unit-dose containers.
TABL...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com