HA-1 epitopes and uses thereof
a technology of ha1 and epitope, applied in the field of biotechnology, can solve the problems of not being able to identify amino acids, and no one has succeeded in identification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
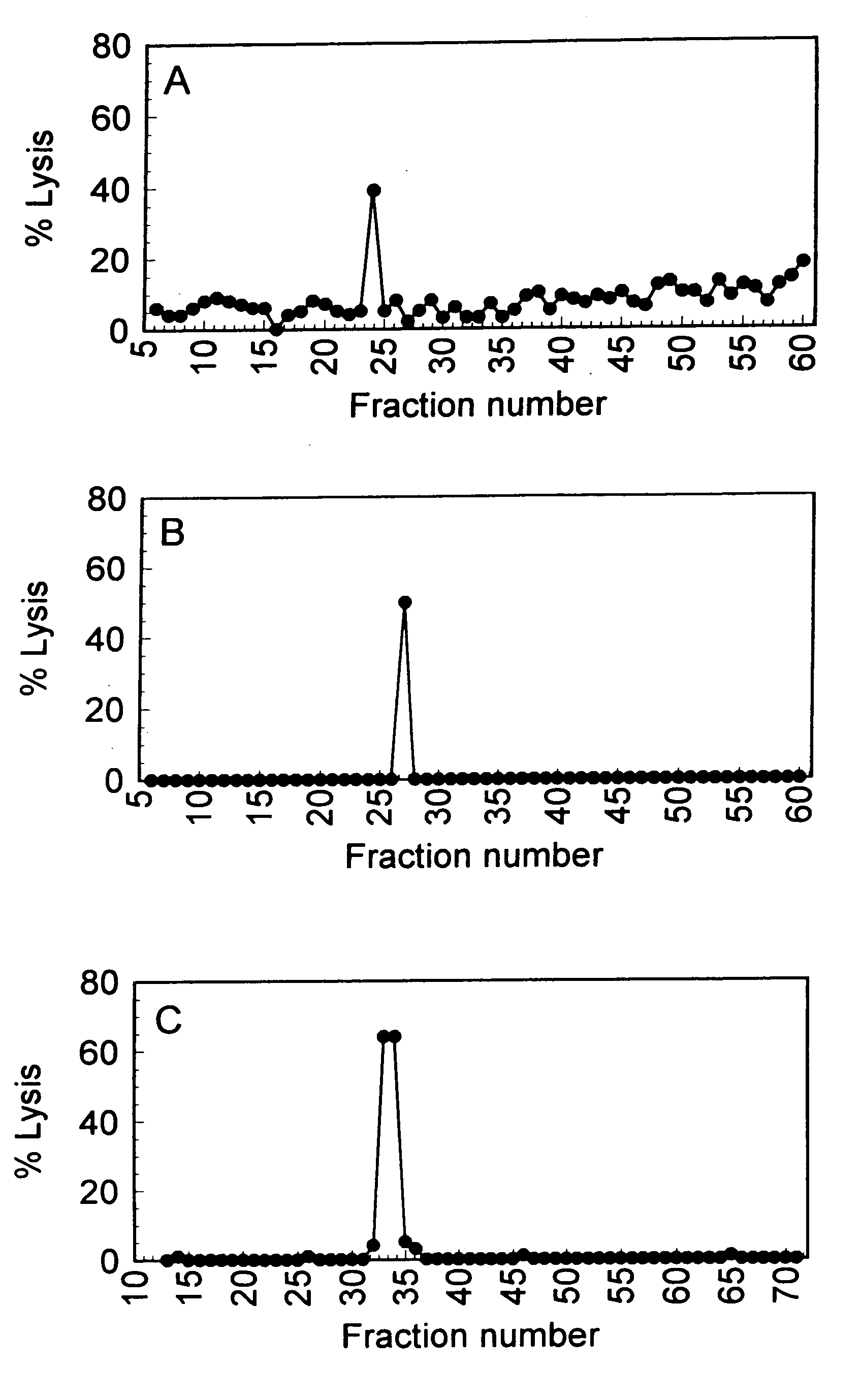
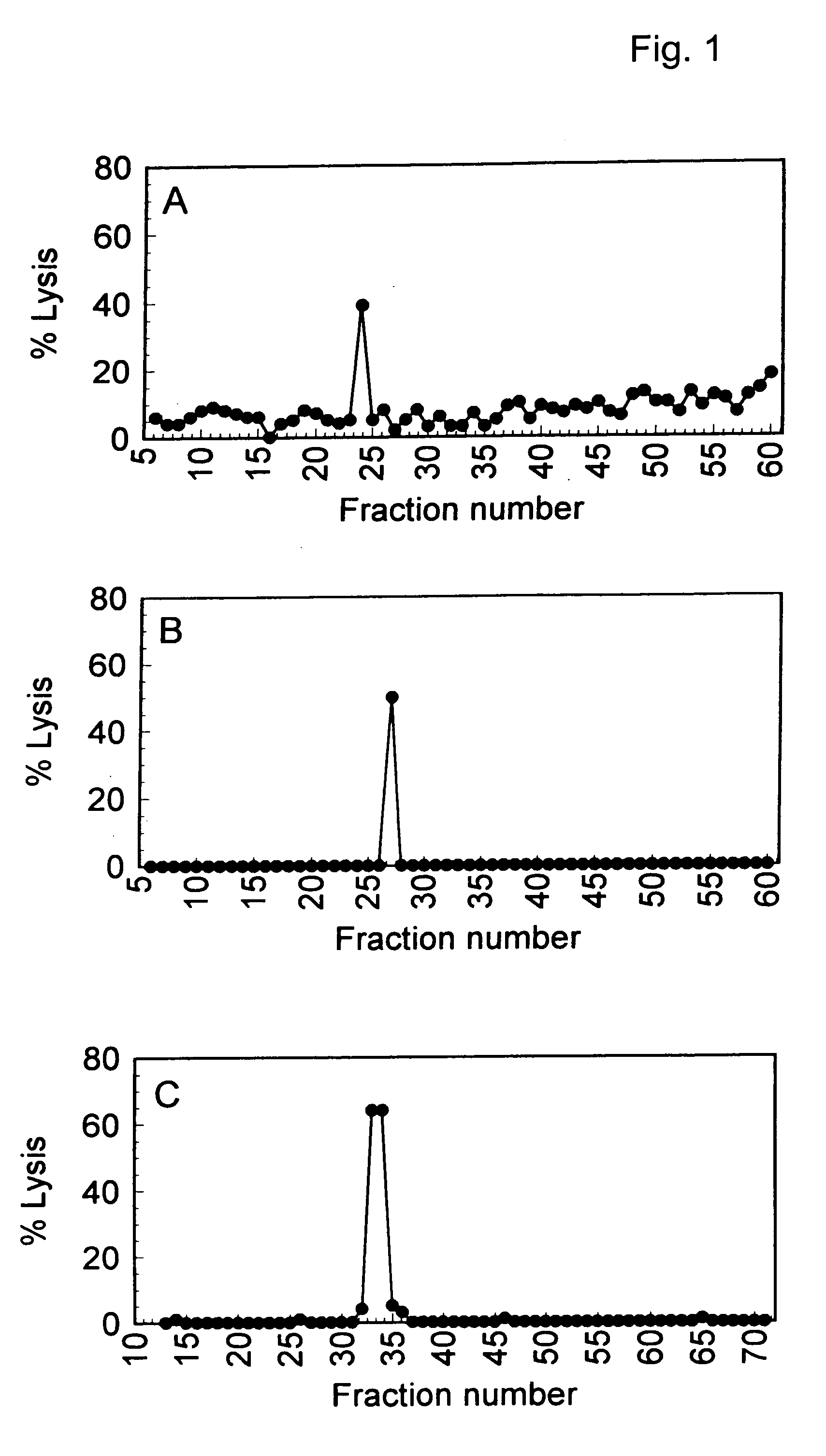
[0115]GvHD is a frequent and life-threatening complication after allogeneic HLA-identical bone marrow transplantation (BMT). Recipients of HLA-identical bone marrow develop acute or chronic GvHD in respectively 36% and 49%.1, 2 Disparities in genes other than the MHC, referred to as minor histocompatibility antigens (mHags), are clearly involved in the development of GvHD after HLA-identical BMT. A recent retrospective analysis revealed the significant association between mismatching for the mHag HA-1 and the induction of GvHD after HLA-identical BMT.3 mHags are recognized by MHC restricted T-cells and were shown to be peptides derived from intracellular proteins presented by MHC molecules.4-6 Here we report the first identification of a polymorphic gene encoding an human mHag. The GvHD-associated mHag HA-1 is a nonapeptide derived from the di-allelic KIAA0223 gene. The HA-1 allelic counterpart encoded by the KIAA0223 gene differs only at one amino acid from the mHag HA-1. Family st...
example 2
[0151]To confirm the hematopoietic system restricted tissue distribution, earlier analyzed by HA-1-specific CTLs, HA-1 mRNA levels were analyzed by quantitative real-time PCR (Example 4) in eight different hematopoietic and six different non-hematopoietic cell types. Only cells of hematopoietic origin expressed significant levels of the HA-1 gene (FIG. 8). No significant HA-1 gene expression was detected in cells of non-hematopoietic origin: i.e., keratinocytes, dermal fibroblasts, proximal tubular epithelial cells (PTECs), human umbilical vein endothelial cells (HUVECs), melanocytes and SV 40 immortalized breast cell lines HaCaT and HBL 100 (FIG. 8).
[0152]Next, we investigated the HA-1 gene transcription levels in 35 epithelial tumor cell lines derived from different carcinomas (Table 1). The HA-1 gene transcription, analyzed by quantitative real time RT-PCR, revealed significant HA-1 mRNA in 26 out of the 35 cell lines of various malignant origins. Table 1 also lists the results o...
example 3
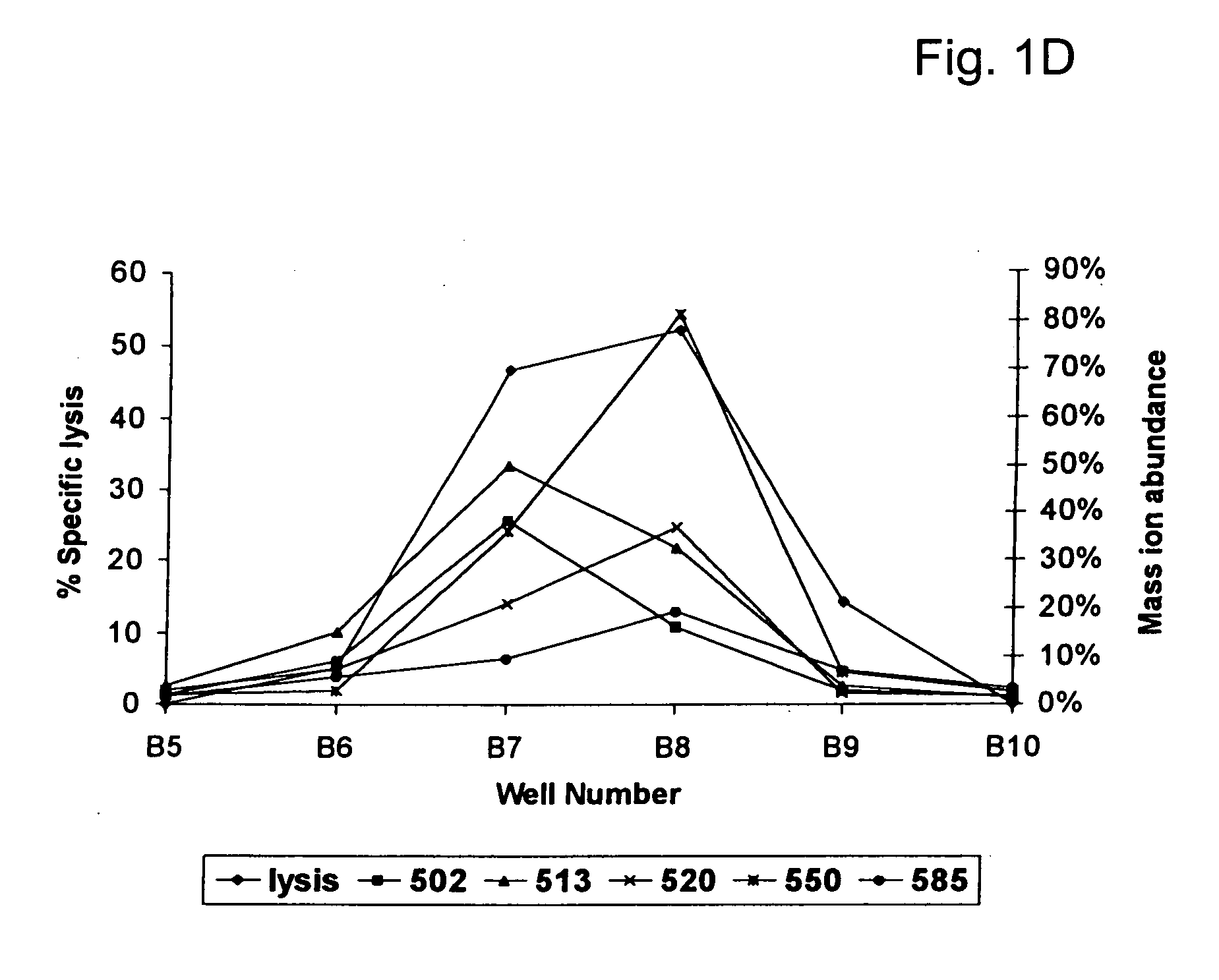
[0156]We have investigated whether the HA-1H / R polymorphic region contains peptides that can be presented by other HLA molecules than HLA-A2. Hereto, we analyzed the binding capacities of HA-1 polymorphic peptides to nine HLA-A and -B molecules that have a frequency of more than 10% in the Caucasian population. Nonameric HA-1H / R peptides (n=18) were tested for binding to these frequent HLA alleles. The peptide binding analyses were extended with two decameric HA-1H / R peptides that contained binding motives for HLA-A3 and with five nonameric / decameric peptides that were predicted to bind to HLA-B14 or to -B60. Next to the binding studies, cellular processing was executed by in vitro proteasome digestion of 29 amino acid long HA-1H and HA-1R peptides. To enlarge the patient population for HA-1-specific immunotherapy, the HLA-B60 binding peptides were analyzed for their in vitro immunizing potential. Hereto, peptide loaded dendritic cells (DCs) were used to induce T-cell responses from...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com