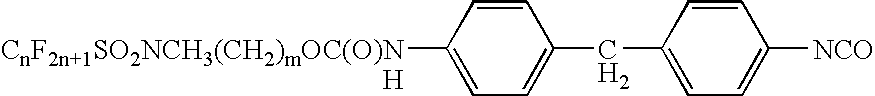
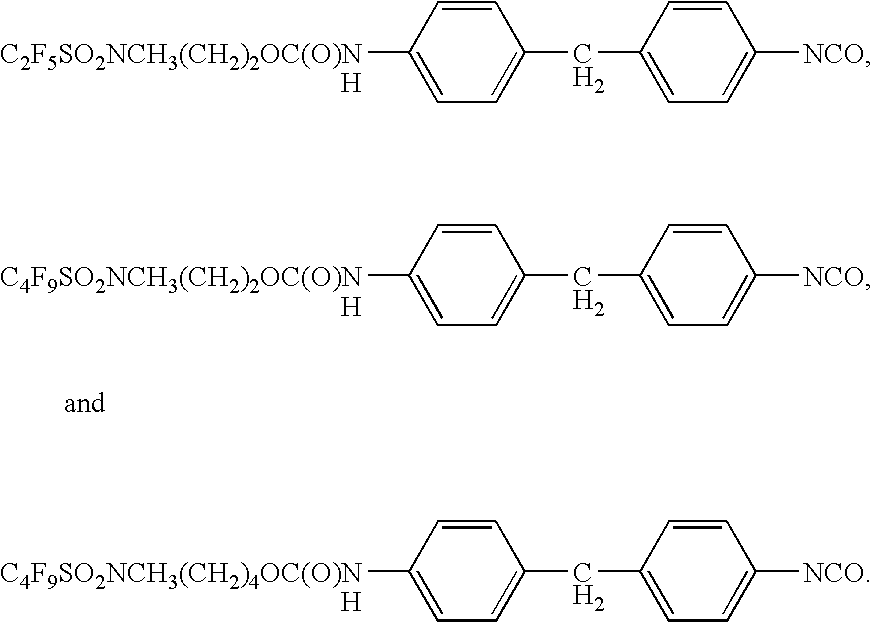
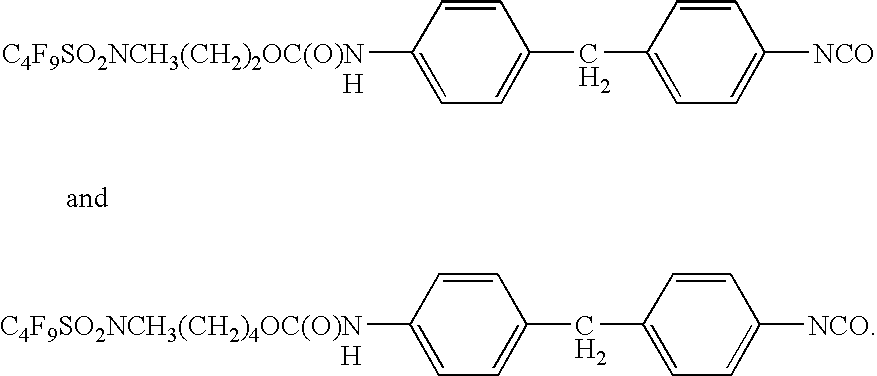
Stain and fouling resistant polyurea and polyurethane coatings
a technology of polyurea and coatings, applied in the field of stain and fouling resistant coatings, can solve the problems of reducing efficiency, affecting the effect of abrasion resistance, and affecting the effect of abrasion resistance, and achieves the effects of high hardness, chemical resistance, and flexibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0057] A two component polyurea (Part A and Part B) was formulated as follows. Part A contained hexamethylene diisocyanate (85.2% by weight, obtained from Rhodia, Inc., Cranbury, N.J., under the trade designation “TOLONATE™ HDT LV2”), glass microspheres (13.5% by weight, obtained from 3M Company under the trade designation “3M™ GLASS MICROSPHERES K37”) and a modified polyurea (1.3% by weight, obtained from BYK Chemie, Wesel, Germany, under the trade designation “BYK™ 410”). Part B contained diethyltoluenediamine (31.6% by weight, obtained from Albemarle Corporation, Bayport, Tex., under the trade designation “ETHACURE 100”), polyoxypropylenediamine (38.7% by weight, obtained from Huntsman Corporation, Salt Lake City, Utah, under the trade designation “JEFFAMINE™ D-2000”), an aromatic secondary diamine (6.3% by weight, obtained from UOP, A Honeywell Company, Tonawanda, N.Y. under the trade designation “UNILINK™ 4200”), a trifunctional amine (2.4% by weight, obtained from Huntsman Cor...
example 2
[0059] A two component polyurea (Part A and Part B) was formulated as follows. Part A contained hexamethylene diisocyanate (84.4% by weight, obtained from Rhodia, Inc., Cranbury, N.J., under the trade designation “TOLONATE™ HDT LV2”), glass microspheres (12.3% by weight, obtained from 3M Company under the trade designation “3M™ GLASS MICROSPHERES K37”), a modified polyurea (1.3% by weight, obtained from BYK Chemie, Wesel, Germany, under the trade designation “BYK™ 410”) and a fluorochemical urethane (2% by weight, obtained from 3M Company under the trade designation “SRC-220”. Part B contained diethyltoluenediamine (32.4% by weight, obtained from Albemarle Corporation, Bayport, Tex., under the trade designation “ETHACURE™ 100”), polyoxypropylenediamine (39.6% by weight, obtained from Huntsman Corporation, Salt Lake City, Utah under the trade designation “JEFFAMINE™ D-2000”), an aromatic secondary diamine (6.5% by weight, obtained from UOP, A Honeywell Company, Tonawanda, N.Y. under ...
example 3
[0061] A two component polyurea (Part A and Part B) was formulated as follows. Part A contained hexamethylene diisocyanate (76.8% by weight, obtained from Rhodia, Inc., Cranbury, N.J., under the trade designation “TOLONATE™ HDT LV2”), glass microspheres (12.2% by weight, obtained from 3M Company under the trade designation“3M™ GLASS MICROSPHERES K37”), a modified polyurea (1.0% by weight, obtained from BYK Chemie, Wesel, Germany, under the trade designation “BYK 410”) and a fluorochemical urethane (10% by weight, obtained from 3M Company under the trade designation “SRC-220”. Part B contained diethyltoluenediamine (32.4% by weight, obtained from Albemarle Corporation, Bayport, Tex., under the trade designation “ETHACURE™ 100”), polyoxypropylenediamine (39.6% by weight, obtained from Huntsman Corporation, Salt Lake City, Utah under the trade designation “JEFFAMINE™ D-2000”), an aromatic secondary diamine (6.5% by weight, obtained from UOP, A Honeywell Company, Tonawanda, N.Y., under ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com