Mechanical and acoustical suspension coating of medical implants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0110]Coronary stents are coated with a polymeric coating solution in accordance with the present invention.
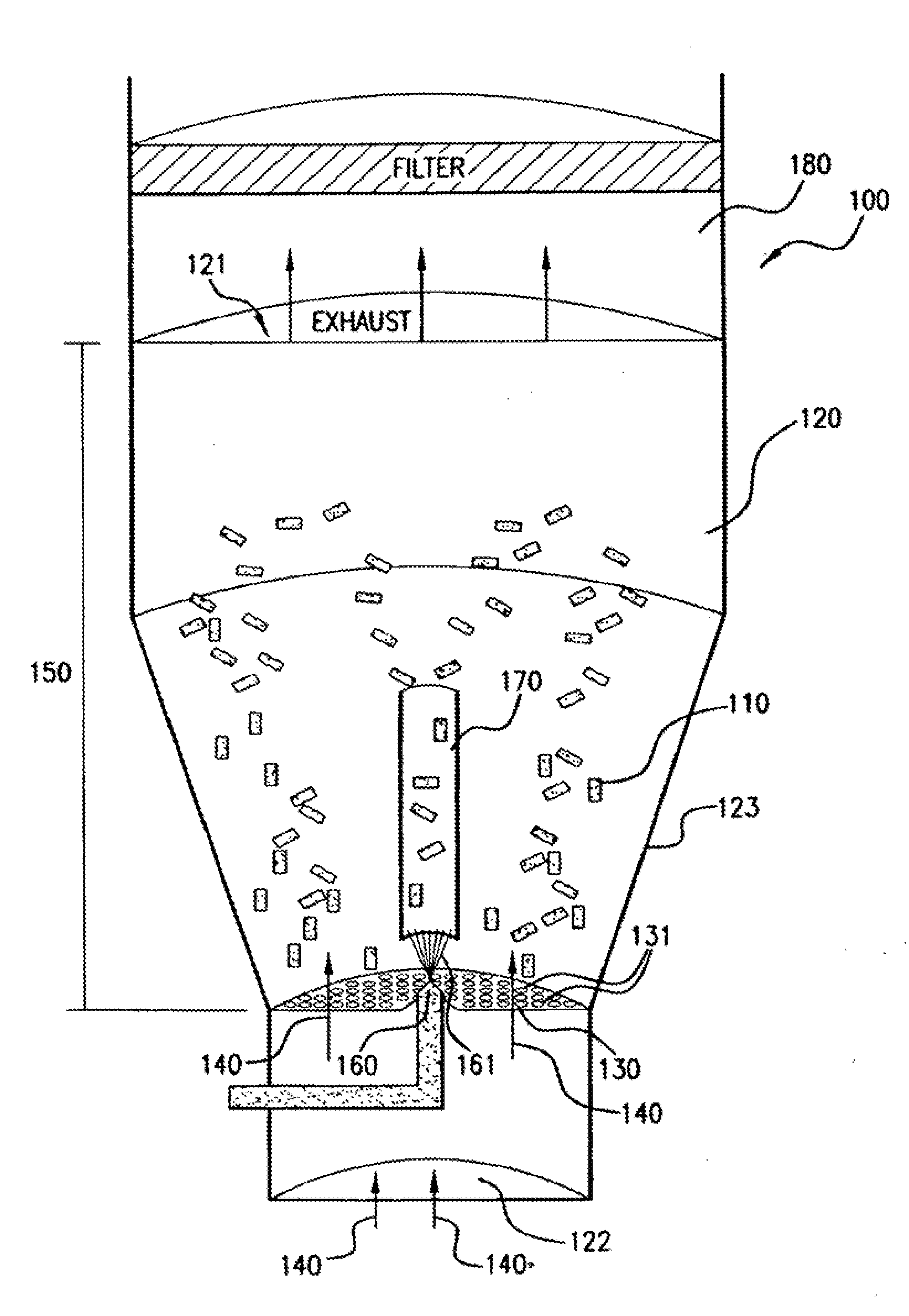
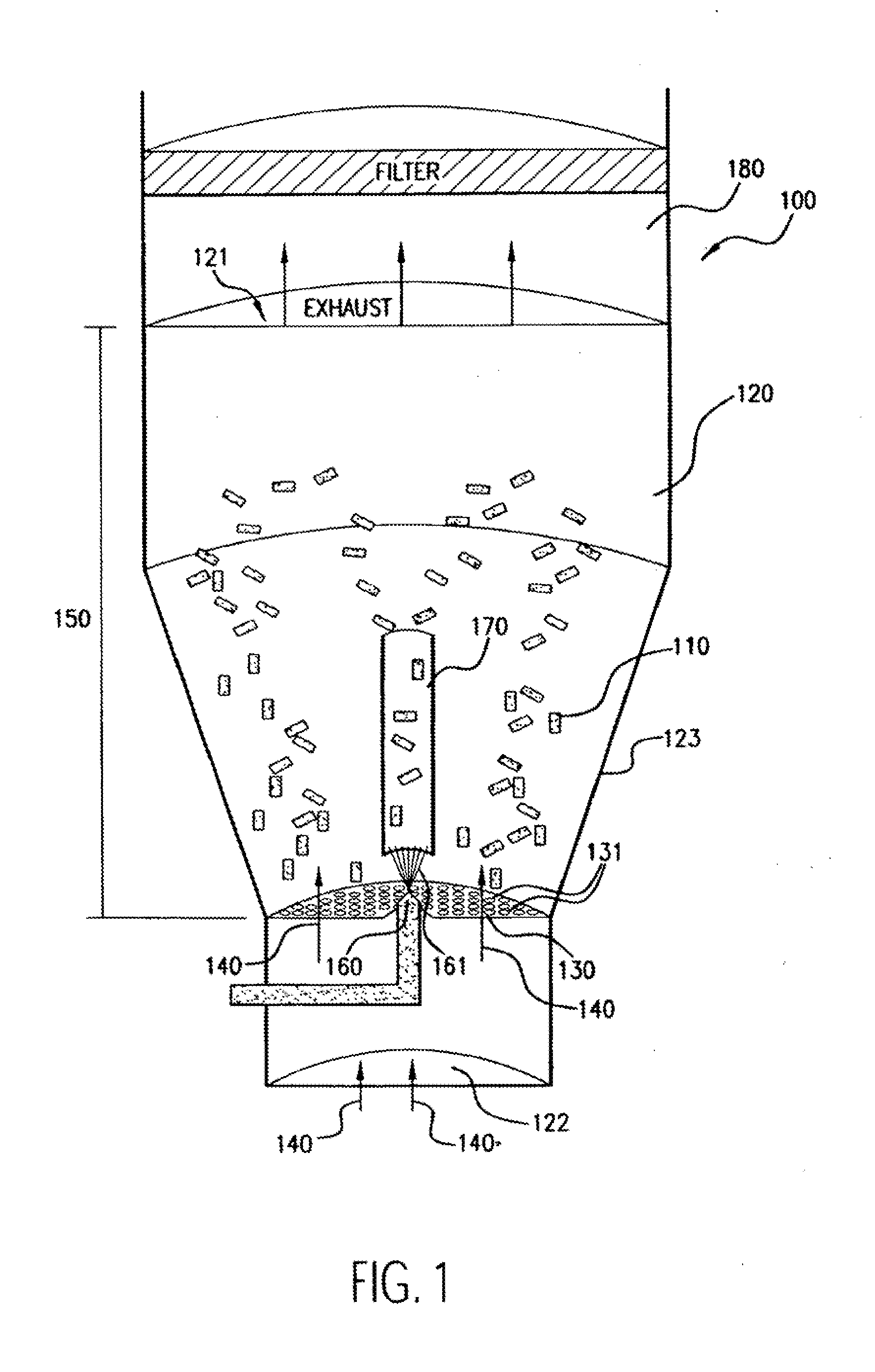
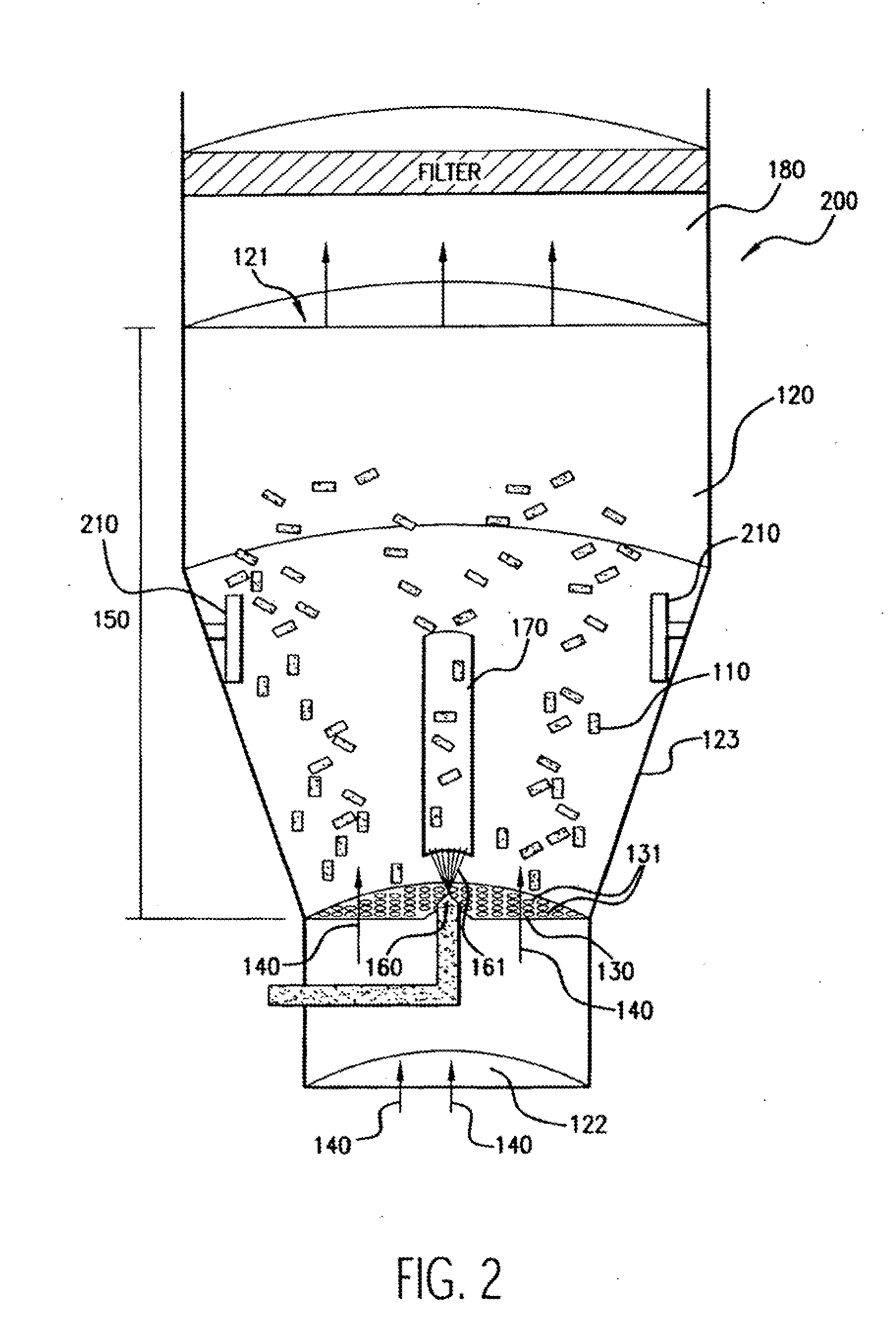
[0111]Numerous (approximately 300 to 600 in this example) NIR stents (Medinol, Tel Aviv) are placed in a Wurster fluidized bed chamber, such as a GPCG-1 (available from Glatt Air Techniques, Ramesey, N.J.). The stents are each about 9 mm-32 mm in length, about 1.5 mm-3.0 mm in diameter, about 7 mg-35 mg in weight, and about 46-200 mm in surface area.
[0112]A coating solution of polyurethane is prepared by mixing the following components (in approximate weight percentages): 0.5-1.0% Corethane 50D (Corvita, Miami, Fla.), 1.0-10.0% dimethylacetamide, and balance tetrahydrofuran. The solution components are mixed using a magnetic stirrer for at least about 8 hours to form a solution or dispersion, which is thereafter filtered with a 1.0 micron Teflon filter.
[0113]The stents are suspended using fluidizing air at about 2-20 psi, at a temperature of about 20-90 C and a dew point of ab...
example 2
[0116]Coronary stents are coated with a layer that comprises both polymeric and drug coating materials in accordance with the present invention.
[0117]NIR® stents are placed in a Wurster fluidized bed chamber, as described in Example 1. A coating solution is prepared by mixing the following components (in approximate weight percentages): about 0.5-2.0% Elvax 40W (available from Dupont, Wilmington, Del.), about 0.05-0.6% paclitaxel, balance chloroform. The coating solution components are mixed with a magnetic stirrer for at least 8 hours to form a solution or dispersion, which is thereafter filtered with a 0.2 micron Teflon® filter.
[0118]The stents are suspended and coated by the processing parameters described in Example 1. The coating process results in stents coated with uniform coating layers in which paclitaxel is evenly distributed on each stent and substantially the same dose applied to every stent in the batch.
example 3
[0119]Coronary stents are coated with multiple polymer coating layers in sequence distributed on each stent and the same dose applied to every stent in the batch in accordance with the present invention.
[0120]NIR® stents are placed in a Wurster fluidized bed chamber, as described in Example 1. A primer coating solution is prepared by mixing the following components (in approximate weight percentages): 0.01-2% Ultrathene UE631-04 (Equistar Chemical, LP, Houston, Tex.) and 99% Chloroform. The stents are suspended and coated by the processing parameters described in Example 1. When the primer coating is completely dry, the stents are further coated with a topcoat solution comprising (in approximate weight percentages): 0.5-0.65% Corethane 50D polyurethane, 1.0-10.0% dimethylacetamide, and balance tetrahydrofuran, prepared by the process described in Example 1.
[0121]The coating process results in stents having uniform, dual-layered coatings. The application of the primer coating enhance...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Force | aaaaa | aaaaa |
| Pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com