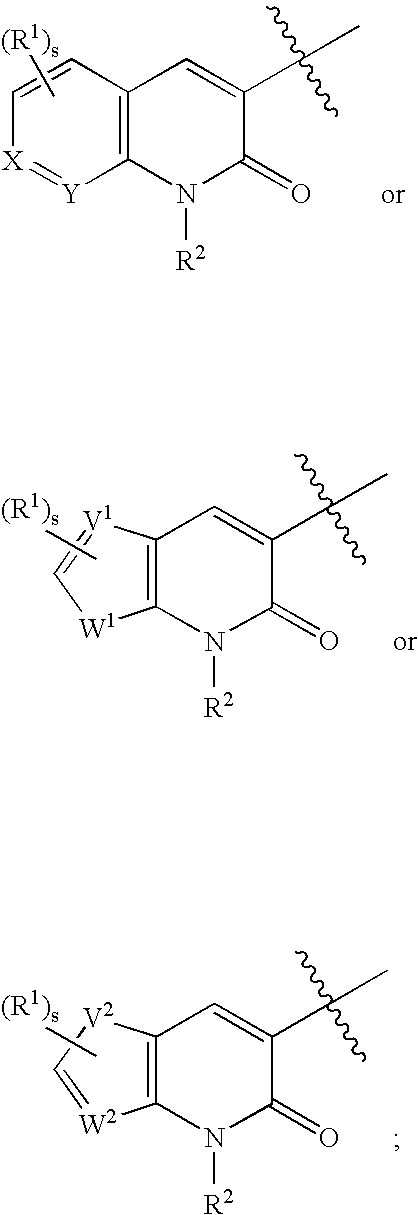
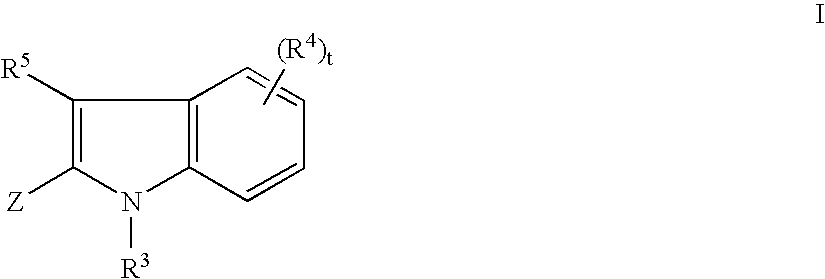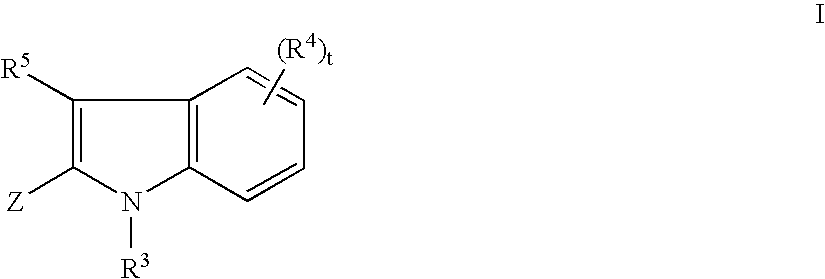Tyrosine kinase inhibitors
a technology of tyrosine kinase and inhibitor, which is applied in the direction of biocide, drug composition, antibody medical ingredients, etc., can solve the problems of visual degeneration, tumor growth is susceptible to antiangiogenic effects,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0309]Examples provided are intended to assist in a further understanding of the invention. Particular materials employed, species and conditions are intended to be illustrative of the invention and not limiting of the reasonable scope thereof.
2-Chloro-3-iodo-quinoline (1-2)
[0310]A suspension of 3-(2-chloro)-quinolineboronic acid (1-1, 5.05 g, 24.3 mmol, 1 equiv, prepared by the method of Marsais, F; Godard, A.; Queguiner, G. J Heterocyclic Chem. 1989, 26, 1589-1594) and N-iodosuccinimide (5.48 g, 24.4 mmol, 1.00 equiv) in acetonitrile (300 mL) was stirred at 23° C. in the dark for 20 h. The reaction mixture was concentrated to dryness, and the resulting yellow solid was partitioned between saturated aqueous sodium bicarbonate solution and dichloromethane. The organic layer was washed with water, then dried over magnesium sulfate and concentrated to give 2-chloro-3-iodo-quinoline as a pale yellow solid. 1H NMR (400 MHz, CDCl3) δ 8.67 (s, 1H), 7.99 (br d, 1H, J=8.4 Hz), 7.75 (br t, 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com