Prostaglandin e2 modulation and uses thereof
a technology of prostaglandin and e2 is applied in the field of prostaglandin e2 change/modulation, which can solve the problems of mental retardation and delayed motor milestones of children affected by the disease, and achieve the effects of reducing the risk of mental retardation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
[0125]Primary human myoblast culture. Normal human myoblasts were derived from a 12-week-old fetus. DM-750, DM-1200 and DM-3200 myoblasts were obtained from the skeletal muscles of 20-, 13- and 15-week-old DM1 fetuses. The length of the CTG repeat expansion in these myoblasts was approximately 750, 1200 and 3200 CTG repeats, respectively, as determined by Southern blot (FIG. 15). Because these myoblasts were obtained from aborted fetuses, it was not determined if they had DM1 or cDM1. All biopsies were obtained in accordance with the Laval University Medical Research Centre ethical committees. Myoblasts were grown in MCDB 120 medium (JRH Biologicals, Lenexa, Kans.) supplemented with 15% heat-inactivated fetal bovine serum, 5 mg / ml insulin, 0.5 mg / ml BSA, 10 ng / ml epidermal growth factor, 0.39 mg / ml dexamethasone, 50 mg / ml streptomycin and 50 mg / ml penicillin (proliferative medium), as previously described (Langlois et al., 2003 Mol. Ther. 7: 670-680; Furling D e...
example 2
CTG Expansion in DM1 Myoblasts
[0139]Because DM1-750 and DM1-1200 myoblasts were obtained from aborted fetuses, it was not clinically determined if they had DM1 or cDM1. The only reliable feature to diagnose the congenital form of the disease at the molecular level is to examine the proximal region of the CTG expansion that was shown to be aberrantly methylated in cDM1 (Steinbach et al., 1998, supra; Filippova et al., 2001. Nat. Genet. 28(4): 335-43). Normal DMPK alleles generate a single 1.8 kb fragment following co-digestion by SacI and HindIII (SI / H) and mutant alleles generate a band augmented by the corresponding length of the CTG expansion. Triple digestion with SacI, SacII and HindIII (SI / SII / H) results in loss of the expansions in normal and in DM1 myoblasts (DM1-750 and DM1-1200) and generate a 1.6 kb fragment (FIG. 15). On the other hand, in cDM1, the expansion is retained in the digested fragment and generate fragments of 11.4 kb, 11.7 kb or 12.9 kb for the cells with 3200...
example 3
Alteration in the Fusion Index of Myoblasts from cDM1 Subjects
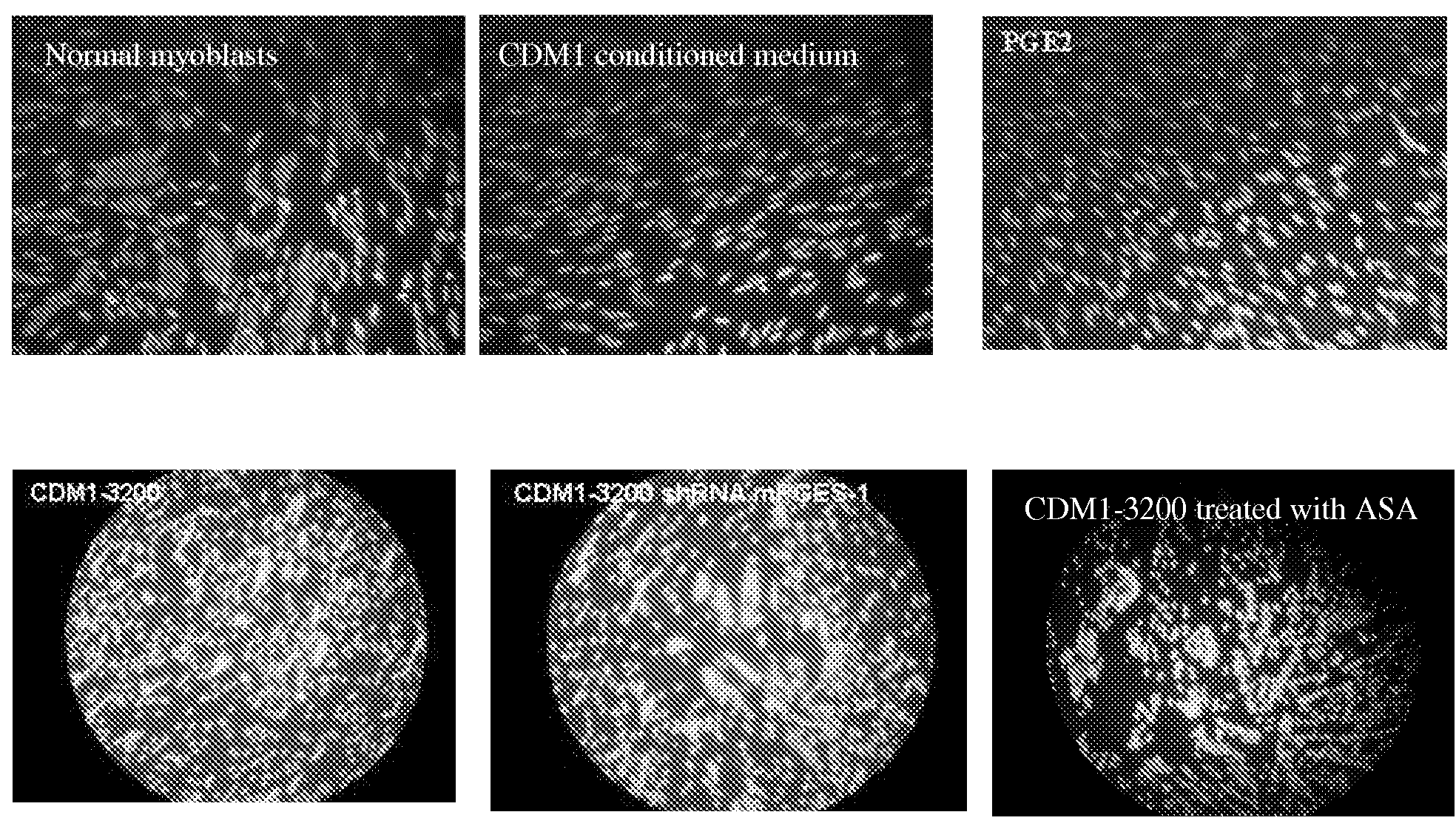
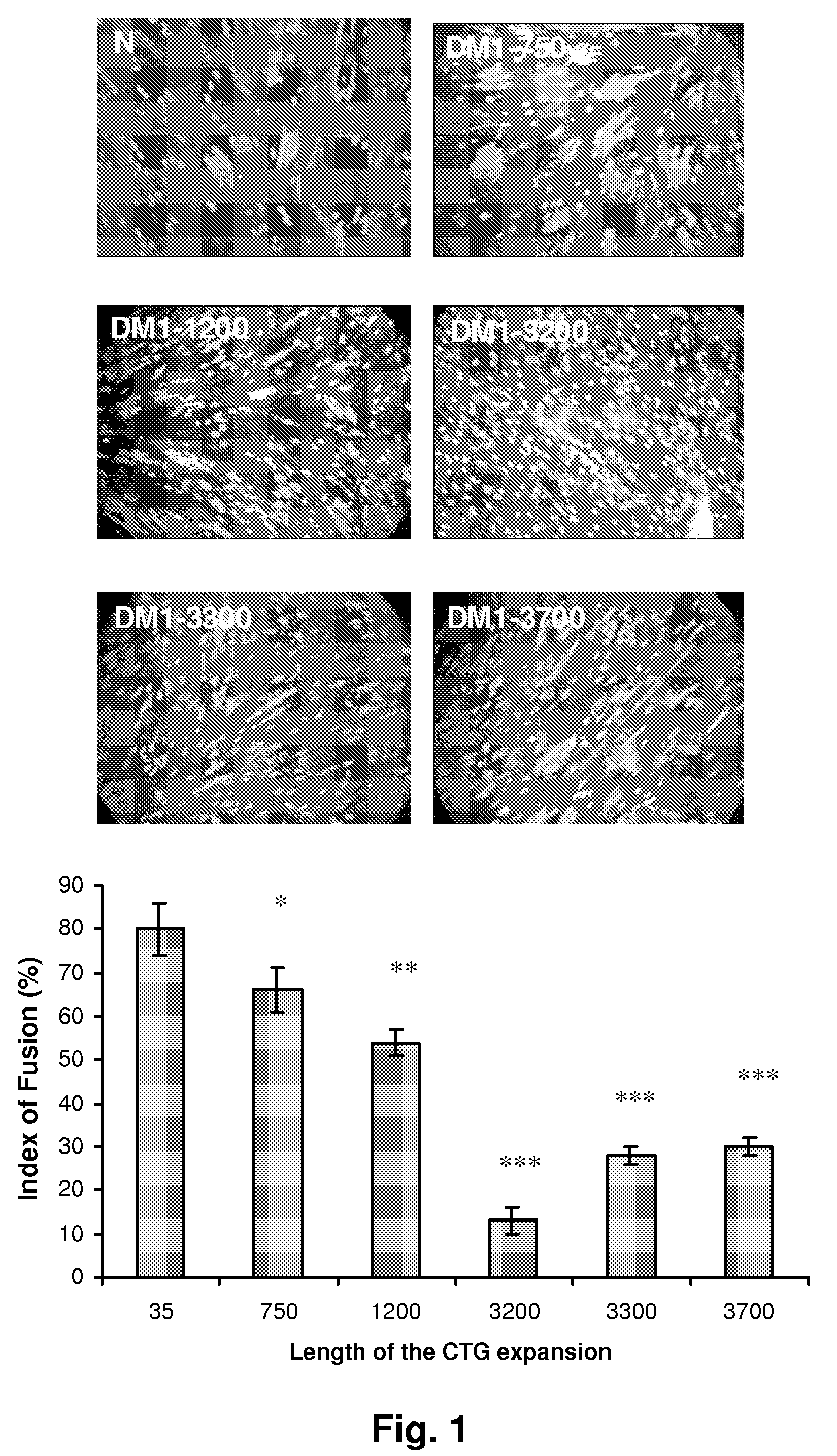
[0140]To investigate the mechanism involved in impaired myogenesis and subsequently to the defective muscle development observed in the severe congenital cDM1, myogenic differentiation of primary myoblasts isolated from cDM1 patients but also from DM1, DM2 and non-affected individual (CON) was assessed in vitro (see FIGS. 1 and 13). Fusion of cDM1 myoblasts associated with large expansion (>3000 CTG) was significantly reduced by 70-80% when compared to DM2 and CON muscle cells (FIG. 13a). A slight decrease of fusion index (25%) was also measured in DM1 cells containing 750 CTG and 1200 CTG suggesting that an additional mechanism may trigger the altered differentiation of cD Ml myoblasts.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal melting point | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| MW | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com