Singlec-9 binding agents
a single-molecule, binding agent technology, applied in the direction of antibody medical ingredients, instruments, peptide/protein ingredients, etc., can solve the problems of anaemia, infection and bleeding, and achieve the effect of facilitating identification and/or diagnosis, effective drug targets, and significantly lower levels of cell-free siglec-9
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0067]The present invention will now be described further by way of example and with reference to the Figures which show:
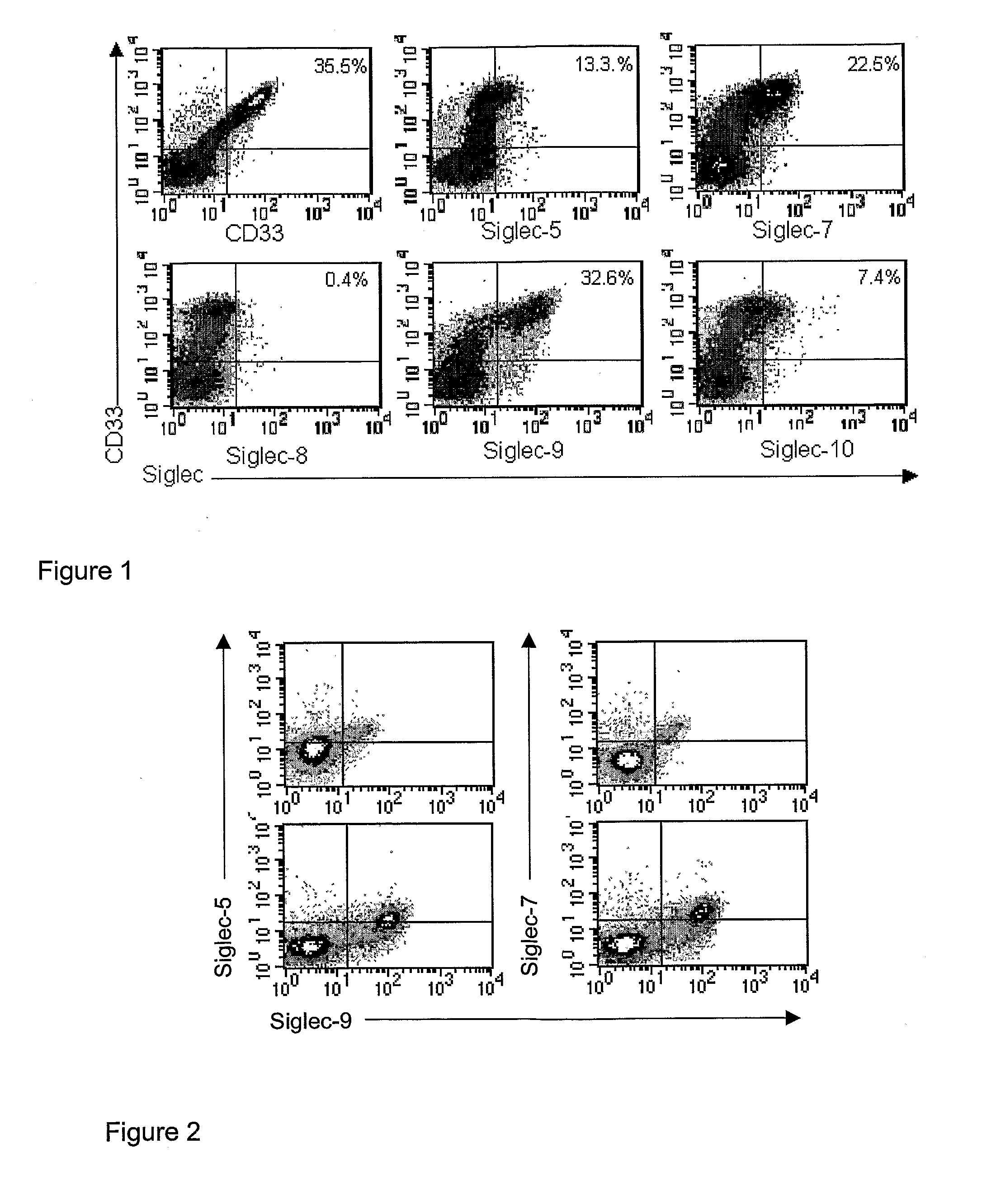
[0068]FIG. 1. Expression of CD33-related Siglecs on AML cells. Mononuclear AML bone marrow cells from sample XXI (see Table 2) were stained with anti-CD33-biotin mAb and the indicated FITC-labelled anti-Siglec mabs, followed by streptavidin-APC and analysed by flow cytometry. The non-viable cells labelled with 7-AAD were not included in the analyses. The left quadrants were set to include more than 99% of cells labelled by the isotype control. The percentages of CD33+ / Siglec+ cells are shown.
[0069]FIG. 2. Co-expression of Siglec-7 and Siglec-5 on the Siglec-9-positive subset of AML cells. Mononuclear AML bone marrow cells from samples I (top panels) and XXI (lower panels) (see Table 2) were stained with anti-Siglec-9-FITC mAb and either anti-Siglec-5-biotin or anti-Siglec-7-biotin mAbs, followed by streptavidin-APC and analysed by flow cytometry.
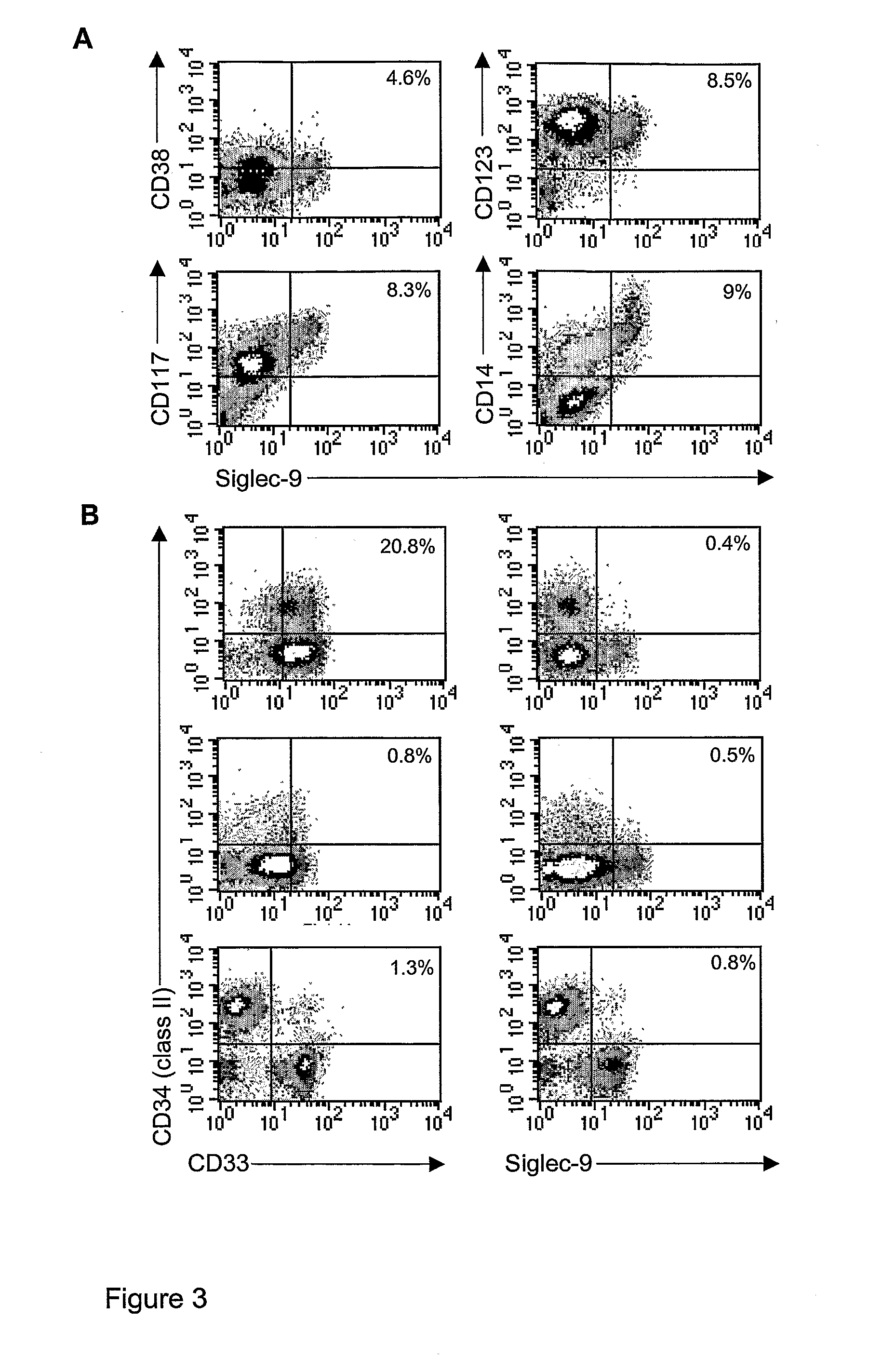
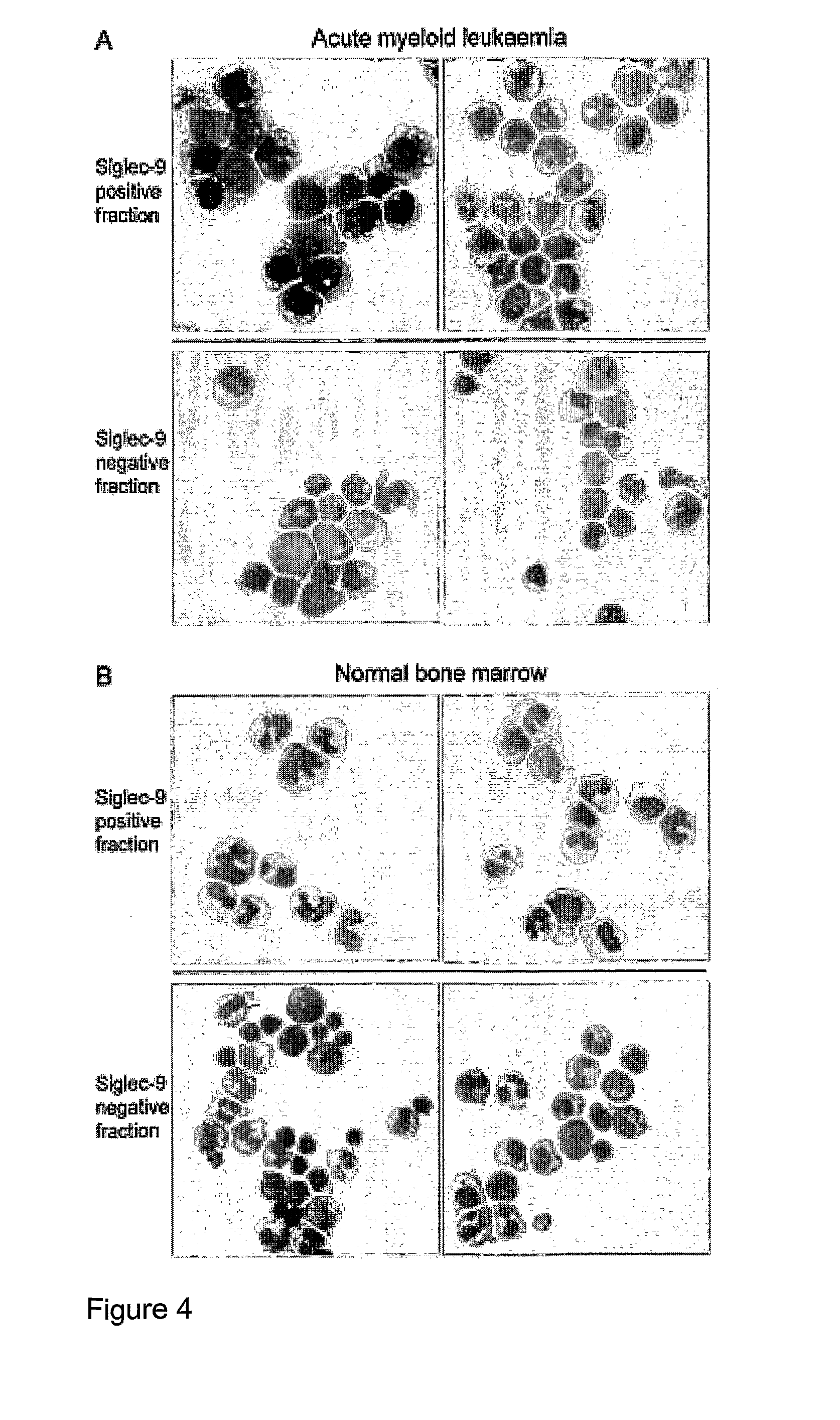
[0070]FIG. 3. Phen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| exposure time | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| differentiation disorder | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com