Visfatin therapeutic agents for the treatment of acne and other conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
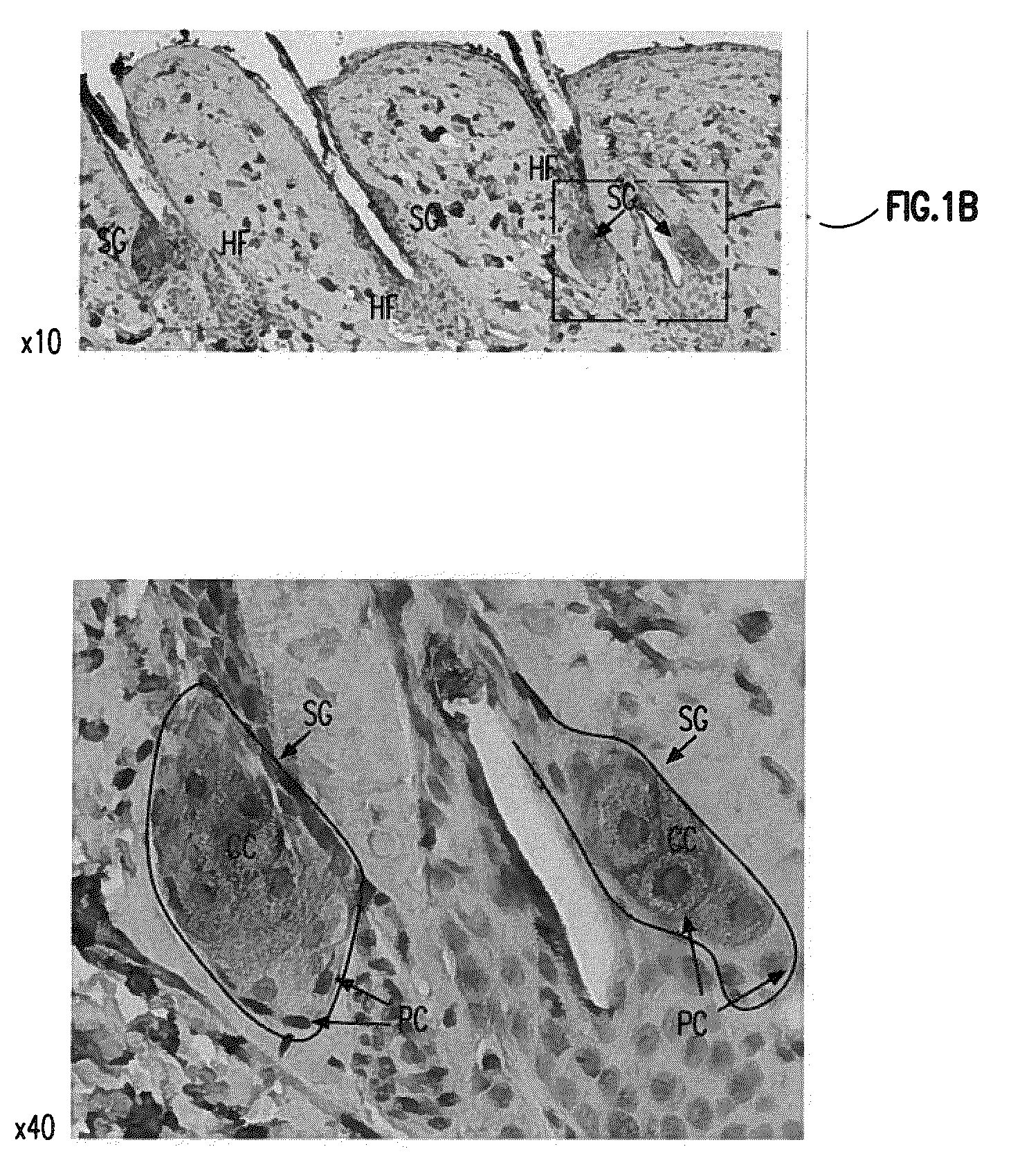
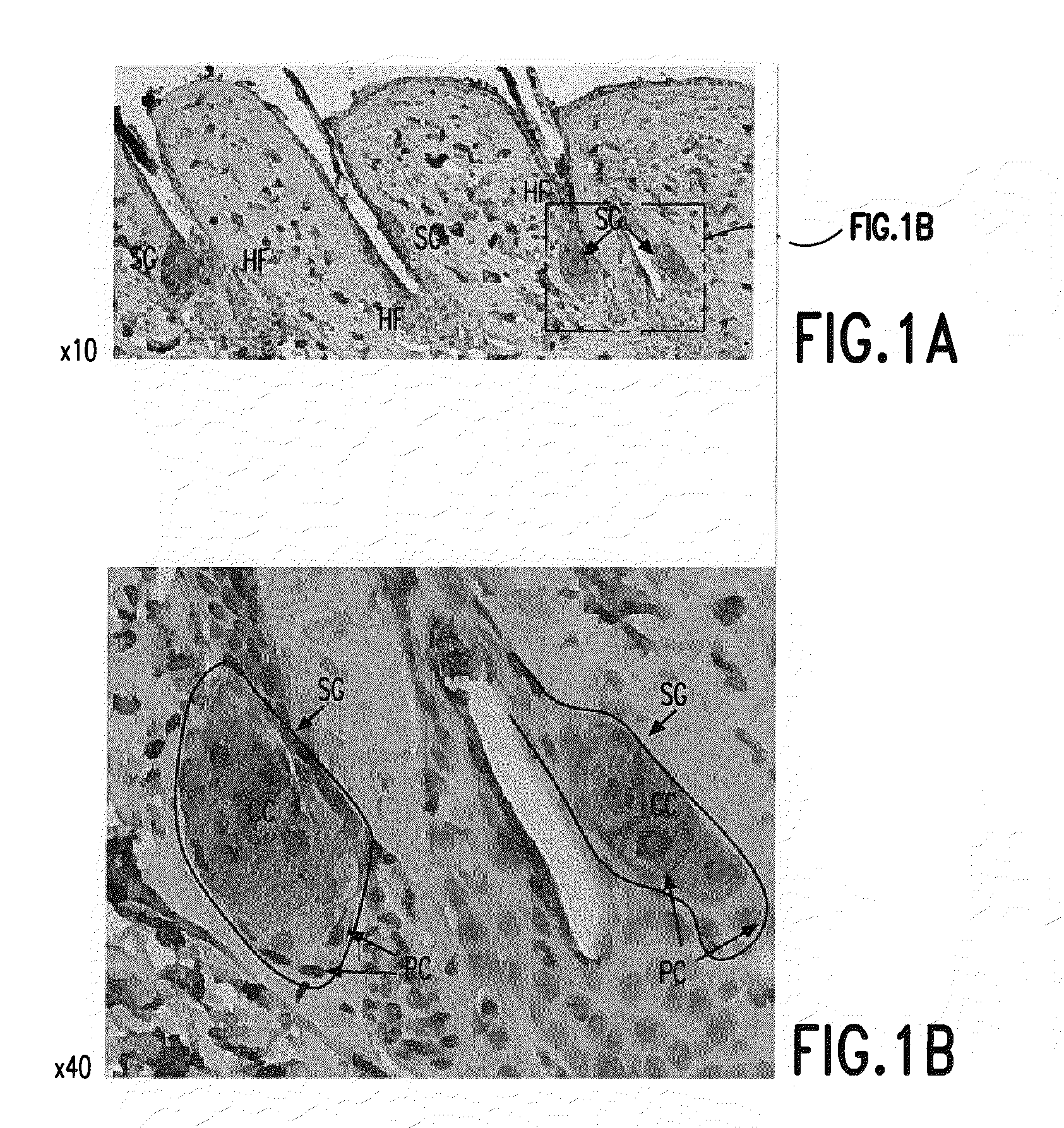
[0144]Visfatin is expressed in the sebaceous glands of the skin. See FIG. 1. Visfatin expression in skin was studied using histological examination. Skin biopsy samples from BalbC mice (Mus musculus) were prepared for histological examination using the materials and methods described above. Visfatin immunohistochemistry was also performed as described above.
[0145]Skin biopsy samples sections were from the skin of 2 month old BalbC mice. Skin samples were fixed in 4% paraformaldehyde and paraffin embedded. Skin sections were specifically stained for visfatin (brown in FIG. 1) using an anti-visfatin antibody as described above. In FIG. 1“HF” means “hair follicle”, “SG” means “sebaceous gland), “PC” means “peripheral cells” and “CC” means “central cells[.]” Images in FIG. 1 represent magnifications of 10× or 40× as indicated and were produced using a Nikon Eclipse 50i microscope.
[0146]As seen in FIG. 1 visfatin is predominantly, and specifically, expressed in the sebaceous glands of th...
example 2
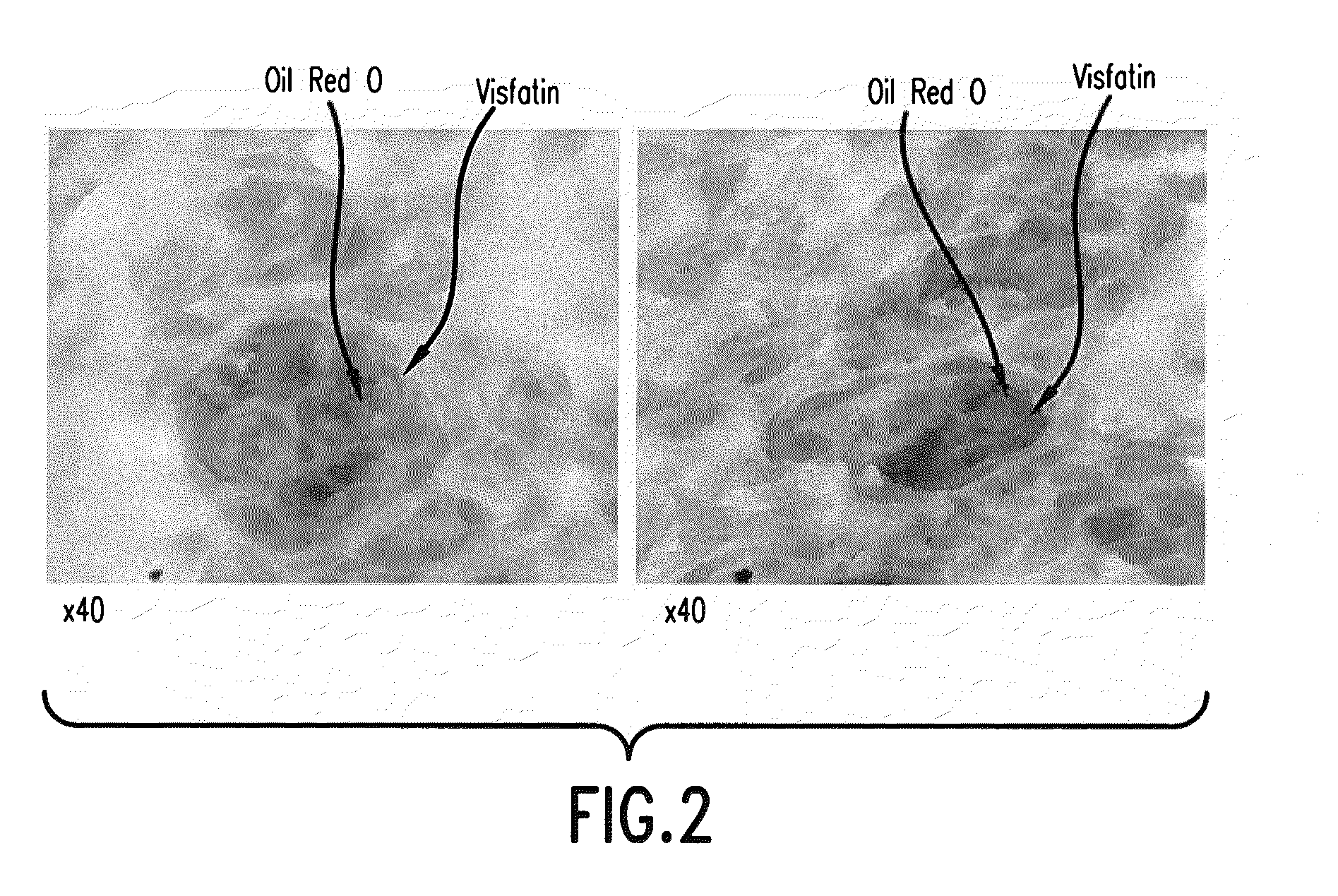
[0147]Visfatin is highly expressed in the sebum accumulating cells present in the interior of the sebum gland. See FIG. 2. Skin biopsy samples from BalbC mice were prepared for histological examination using the materials and methods described above. Visfatin immunohistochemistry and Oil Red O staining was also performed as described above.
[0148]Skin biopsy samples sections were from the skin of 2 month old BalbC mice. Skin samples were fixed in 4% paraformaldehyde and paraffin embedded. Skin sections were specifically stained for visfatin (brown in FIG. 2) using an anti-visfatin antibody as described above. Skin sections were also stained with Oil Red O to identify accumulations of lipids, such as sebum lipids. Images in FIG. 2 represent magnifications of 40× as indicated and were produced using a Nikon Eclipse 50i microscope.
[0149]As seen in FIG. 2 visfatin expression and a large pool of lipids are clearly co-localized within those cells having morphological features characteristi...
example 3
[0150]Topical administration and intradermal administration of visfatin increases the number of sebocytes located inside of the sebaceous glands. See FIG. 3.
[0151]A stock preparation of recombinant, Mus musculus visfatin (Enzo Life Sciences Inc., Farmingdale, N.Y., USA) was prepared in 0.1 M ammonium bicarbonate buffer solution. This stock solution was then used to prepare a topical solution comprising 0.01 μg / ml of recombinant, Mus musculus visfatin in PBS. The stock solution was also used to prepare an intradermal solution comprising 0.01 μg / ml of recombinant, Mus musculus visfatin in PBS containing 0.1% (v / v) DMSO. Adult BalbC mice having an average body weight of 25 g then received either 200 μL of the topical solution by topical administration to the skin treatment area and the use of sterile gauze or 200 μL of the intradermal solution by injection into the skin treatment area. Mice were treated in this fashion with the topical solution or intradermal solution delivered once da...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com