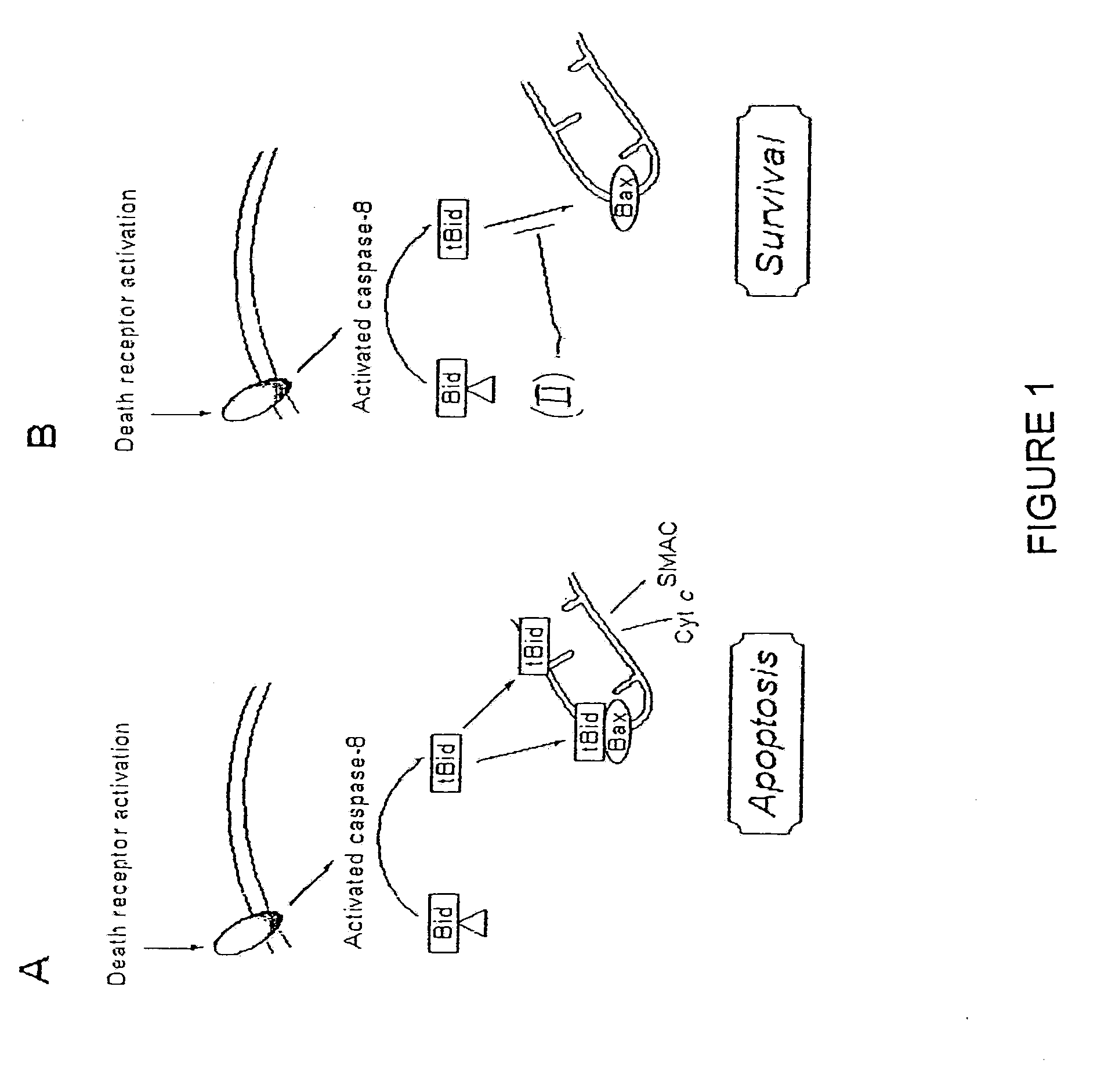
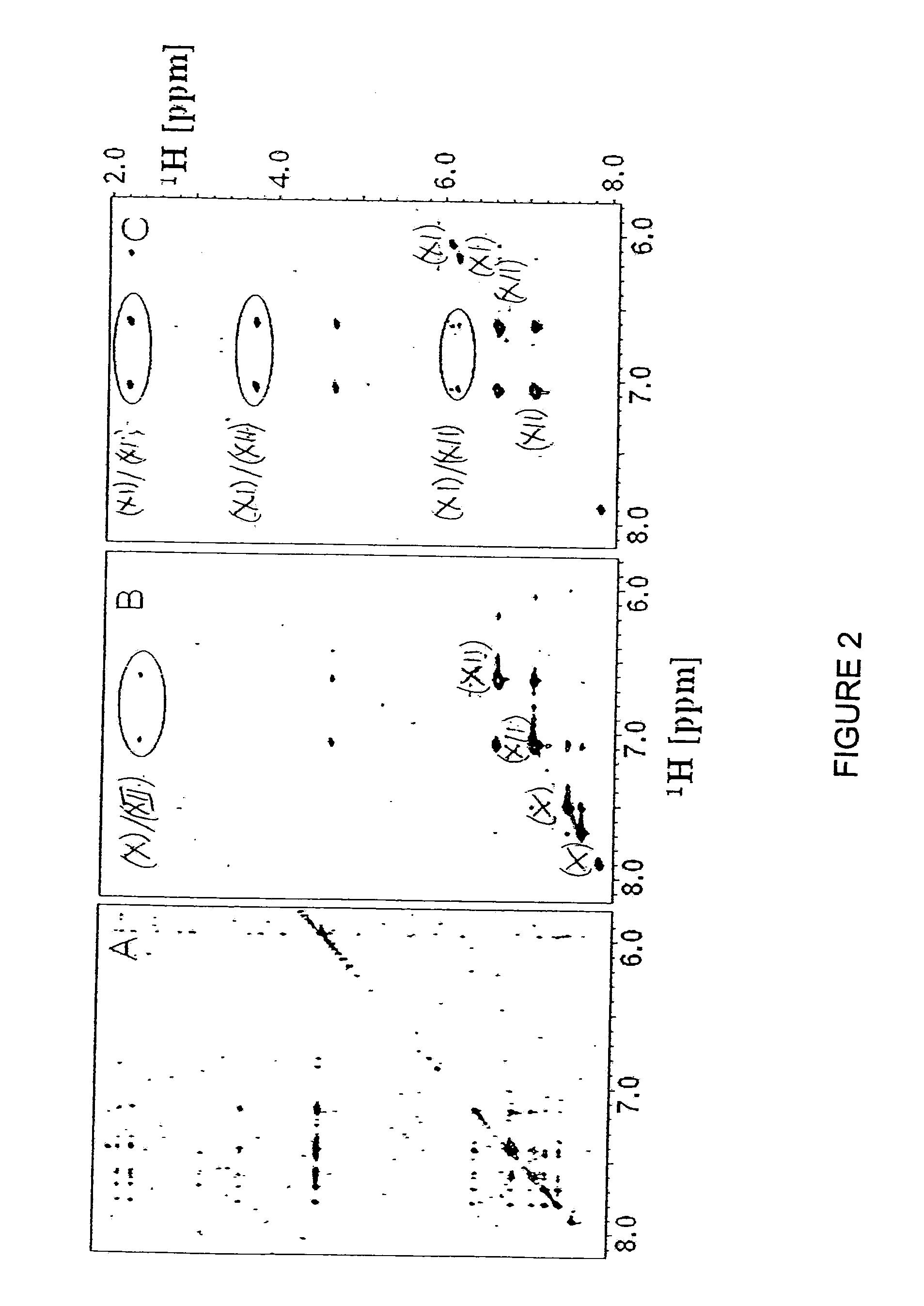
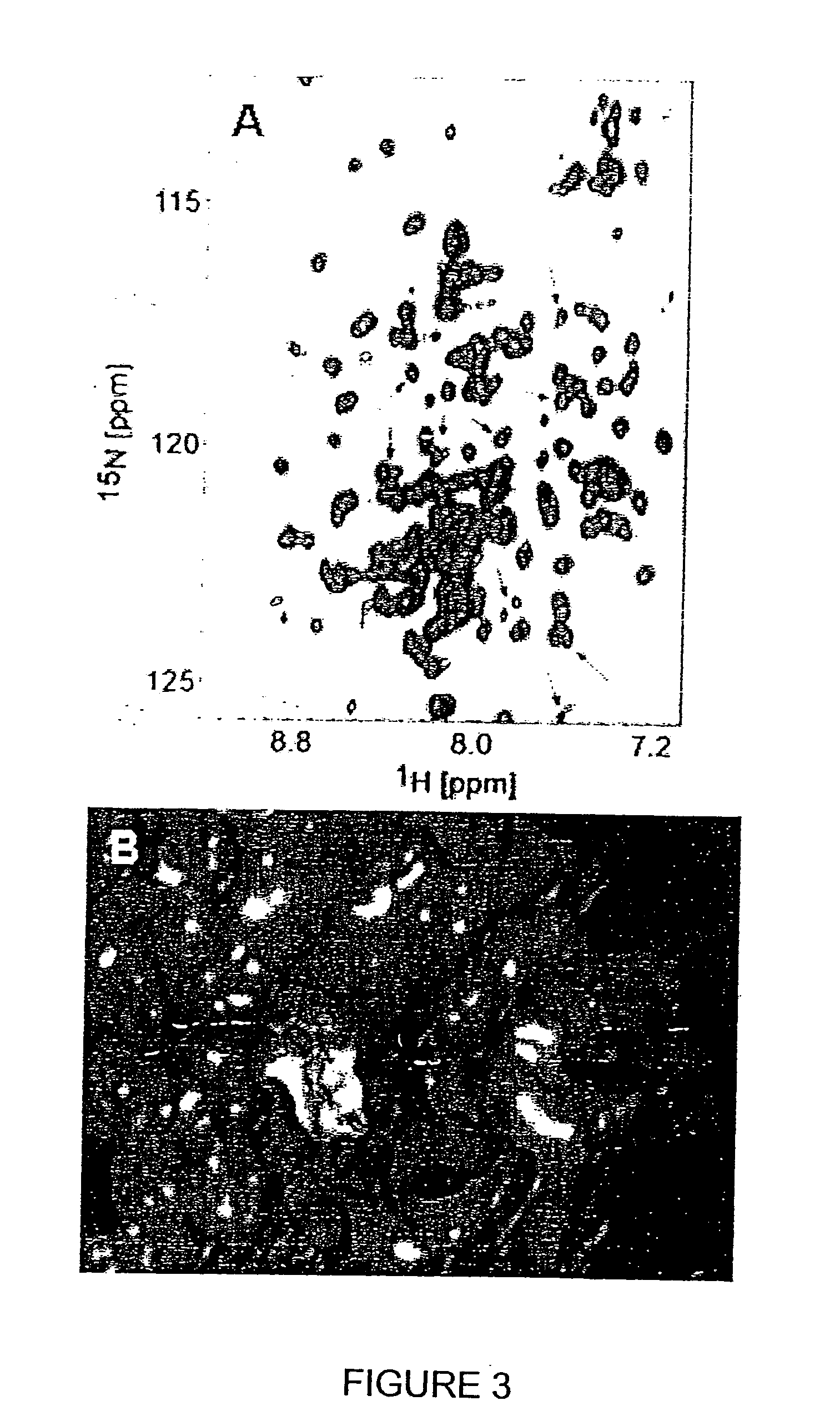
Inhibition of Bid-Induced Cell-Death Using Small Organic Molecules
a small organic molecule and cell death technology, applied in the field of compounds, can solve the problems of cell death, no such therapeutic agent has been developed,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
General Materials and Methods
Library Design
[0084]A library design approach was used. The NMR compound library was composed of about 300 low molecular weight compounds representing diverse core structures. This library was assembled and individual 1D 1H spectra were measured in D2O buffer as control of compound purity, stability, and solubility in water buffer. The compounds having reactive functional groups such as halides, anhydrides, epoxides, aziridines, phosphonates and sulphonates esters, imines, aldehydes, Michael acceptors, halopyrimidines, were not included in the library. The following criteria were adopted for selection of the compounds for the library: average molecular weight was less than 300 Daltons; octanol / water repartition coefficient (LogP) was less than 1.3; number of rotatable bonds was between 0 and 2. The library was designed to optimize the detection of trNOEs and ILOEs by selecting compounds with appropriate derivatization of functional groups with proton NMR...
example 2
Synthesis of N-((4-(4-aminophenylthio)phenylcarbamoyl)methyl)-2,4-dihydroxybenzamide (Compound I)
[0092]
[0093]The synthesis of the title Compound I is shown schematically on FIGS. 5A and 7. Briefly, the formation of the peptide bond was aided by resin-bound carbodiimide, such as N-cyclohexylcarbodiimide-N′-propylmethyl polystyrene (PS-CDT) (available from) Argonaut Technologies) or 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride (WS-CDI resin), using as starting materials the commercially available 4-amino-4′-nitrodiphenyl sulfide (available from Aldrich) and 4-[(tert-butoxycarbonyl)amino]butanoic acid (Boc-GABA-OH, available from Novabiochem). Stirring the reaction mixture at room temperature resulted in the corresponding Boc-protected amine (Compound VI, also shown above in the application). De-protection with trifluoroacetic acid (TFA) gave the free amine (Compound VII), with good yield. Following reaction with 4-methoxybenzenesulfonyl chloride afforded the correspondi...
example 3
Synthesis of {3-[4-(4-nitro-phenylsulfanyl)-phenylcarbamoyl]propyl}-carbamic acid tert-butyl ester (Compound VI)
[0095]
[0096]To synthesize the title Compound VI, PS-CDI resin described in Example 2 (730 mg, 1.0 mmol) was added to a dry round bottomed flask. t-Boc-4-aminobutanoic acid (152 mg, 0.75 mmol) was added as a solution in CH2Cl2 (4 ml) and the reaction mixture was stirred at room temperature. After 5 minutes, 4-amino-4′-nitrodiphenyl sulfide (123 mg, 0.5 mmol) in 4 ml of CH2Cl2 was added and the suspension stirred at room temperature for 4 days. The reaction mixture was filtered under vacuum and the resin was washed twice with CH2Cl2. Concentration of the filtrate afforded a crude that was purified by flash chromatography (hexane / ethyl acetate 1:1) to give the pure title Compound VI (274 mg, 64%) as a yellow solid, together with unreacted starting material (25 mg, 20%).
[0097]The following spectral data was obtained for the title Compound VI: 1H NMR (d-DMSO, 500 MHz): 10.19 (s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com