Esters with Antimicrobial, Bioresistant and Fungal Resistant Properties
a technology of antimicrobial, fungal resistance and antimicrobial/antifungal properties, which is applied in the field of esters with bioresistant, fungal resistant and antimicrobial/antifungal properties, can solve the problems of metal working fluid bases, metal working fluids (mwf) and metal working fluid bases suffering a failure mode, destabilizing the emulsion, and being especially acu
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Production of a Metal Working Fluid Base
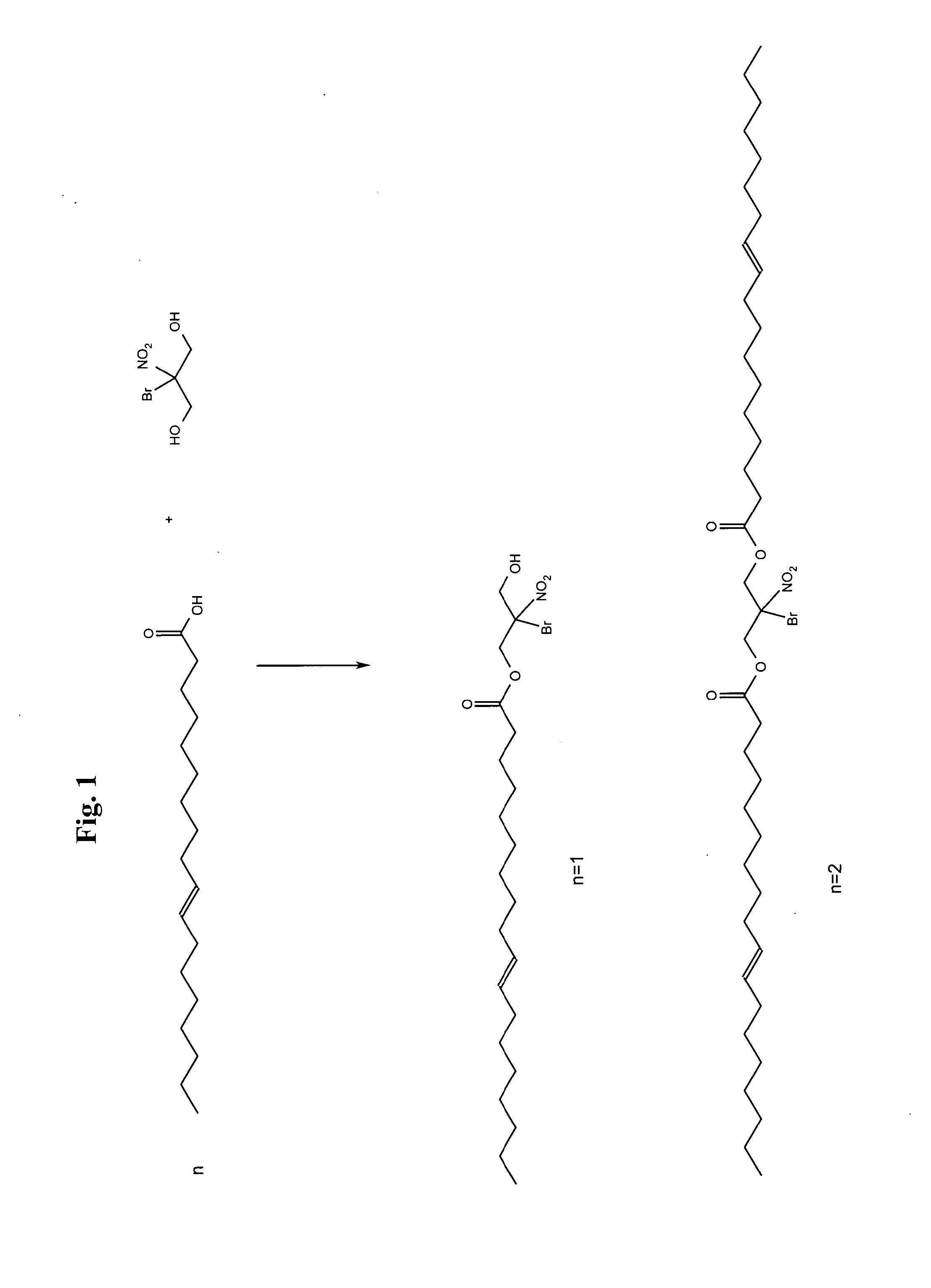
[0031]A vessel equipped with a nitrogen blanket and condenser was charged with 1128 g (4 moles) of oleic acid, 400 g BNPD (2 moles) and 2 g conc. Sulfuric acid. The vessel was heated to 288 F when condensation began. The temperature leveled off between 352 F and 356 F and was kept at temperature for 3 hours. The BNPD dioleate recovered was a dark brownish liquid.
[0032]The dioleate produced was then incorporated into a standard MWF base at 10% (1,300 PPM BNPD) in the concentrate by substituting it for part of the existing ester. The bases were then subjected to ASTM 3946-92, “Standard Test Method for Evaluating the Bacteria Resistance of Water-Dilutable Metal Working Fluids”. On Day 5 of the testing, the BNPD ester containing fluid showed bacterial count of 3×10̂3 CFU / ml, which is considered under control. The control sample had a bacterial count of 1×10̂7, which is not considered under control.
[0033]At an incorporation level of 6% (823 PPM BNP...
example 2
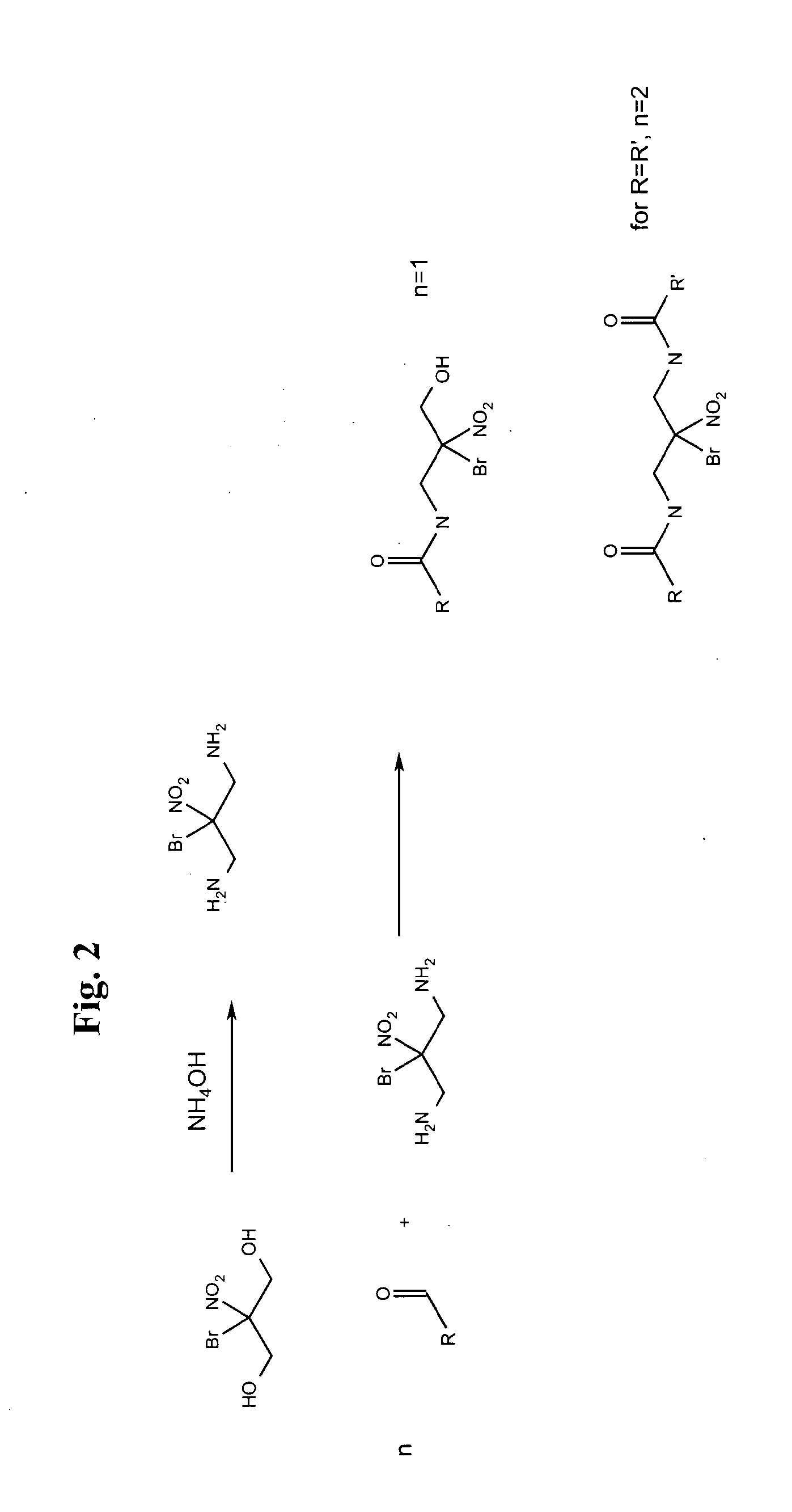
Synthesis of Acid Functional, Pendant Fatty Chain Containing, BNPD Ester
[0035]In a vessel with heat, agitation, condenser, and nitrogen blanketing is charged 400 g (2 moles) of BNPD, 616 g (4 moles) of 1,2-Cyclohexanedicarboxylic anhydride (HHPA) and 150 g xylene as a reflux solvent. The vessel is heated to 323 F at which point the reaction exothermed and began to darken. The temperature was then reduced and held at 302 F for one hour. 1162 g of final product was recovered that was a thick, dark transparent liquid. This product will be referred to as HHPA / BNPD-003.
[0036]In a vessel with heat, agitation, condenser, and nitrogen blanketing is charged 706 g of HHPA / BNPD-003 from above, 806 g of Crisamine PCD-2, 2 mole ethoxylate of primary coco amine, and 150 g xylene as a reflux solvent. The vessel is heated to 350 F for three hours until the theoretical water loss was collected in the trap and the evolution of water stopped. Approximately 1,400 g of a dark, thick translucent liquid w...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| volatile | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com