Adjustable chromophore compounds and materials incorporating such compounds
a technology of chromophore compounds and compounds, which is applied in the field of adjustable chromophore compounds and materials, can solve the problems of chromophore compounds that tend to degrade, and achieve the effects of less absorption, enhanced light absorption of a nucleus portion, and enhanced light absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
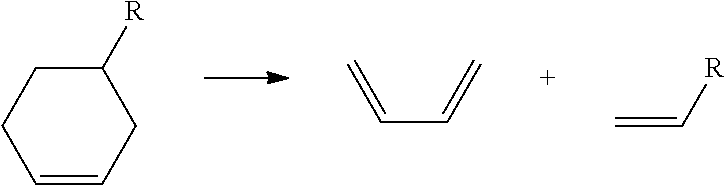
Example 1 above illustrates one exemplary embodiment of the invention. As can be seen, a chromophore compound B—X includes a benzotriazole base chromophore
(B) and an adjustable moiety (X), which is a dicyclopentadiene. The adjustable moiety (X) includes a mobilization inhibiting group (Z), which is preferably an alkane chain, and an electron donating moiety (D), which is preferably an alkoxy group. The base chromophore compound (B) includes an electron withdrawing group (W), which is preferably one of the halogen or halogenated groups discussed above. As can be seen, upon exposure to predetermined radiation, the chromophore compound B—X becomes the chromophore compound B—C and separable group (S) with the chromophore compound B—C having a conjugated double bond and the electron donating group (D) as well as the remaining group (C). The base chromophore (B) then includes the electron withdrawing group (W). Further, the separable group (S) includes the mobilization inhibiting group (Z...
example 2
Example 2 above illustrates another exemplary embodiment of the invention. As can be seen, a chromophore compound B—X includes a benzotriazole base chromophore (B) and an adjustable moiety (X), which is a dicyclopentadiene. The adjustable moiety (X) includes a mobilization inhibiting group (Z), which is preferably an alkane chain, and an electron withdrawing moiety (W), which is preferably a halogen group. The base chromophore compound (B) includes an electron donating group (D), which is preferably an alkoxy group. As can be seen, upon exposure to predetermined radiation, the chromophore compound B—X becomes the chromophore compound B—C and separable group (S) with the chromophore compound B—C having a conjugated double bond and the electron withdrawing group (W) as well as the remaining group (C). The base chromophore (B) then includes the electron donating group (D). Further, the separable group (S) includes the mobilization inhibiting group (Z).
example 3
Example 3 above illustrates another exemplary embodiment of the invention. As can be seen, a chromophore compound B—X includes a benzotriazole base chromophore (B) and an adjustable moiety (X), which is a dicyclopentadiene. In this example, a cyclic moiety of the base chromophore (B) and the dicyclopentadiene share a common bond. The adjustable moiety (X) includes a mobilization inhibiting group (Z), which is preferably an alkane chain. Further, the base chromophore (B) includes an electron withdrawing moiety (W), which is preferably a halogen group and an electron donating group (D), which preferably includes an alkoxy group. As can be seen, upon exposure to predetermined radiation, the chromophore compound B—X become the chromophore compound B—C and separable group (S) with the chromophore compound B—C having a conjugated double bond as the remaining group (C) and the electron withdrawing group (W). The base chromophore (B) also includes the electron donating group (D). Further, t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelengths | aaaaa | aaaaa |
| chemical moiety | aaaaa | aaaaa |
| chemical | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com