Methods of fetal abnormality detection
a technology of fetal abnormality and detection method, applied in combinational chemistry, biochemistry apparatus and processes, library member identification, etc., can solve the problem of high cost of generating sufficient data for fetal aneuploidy detection, and the sequence of a large number of polynucleotides can be expensiv
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
“Hot Spot” Amplification Strategy
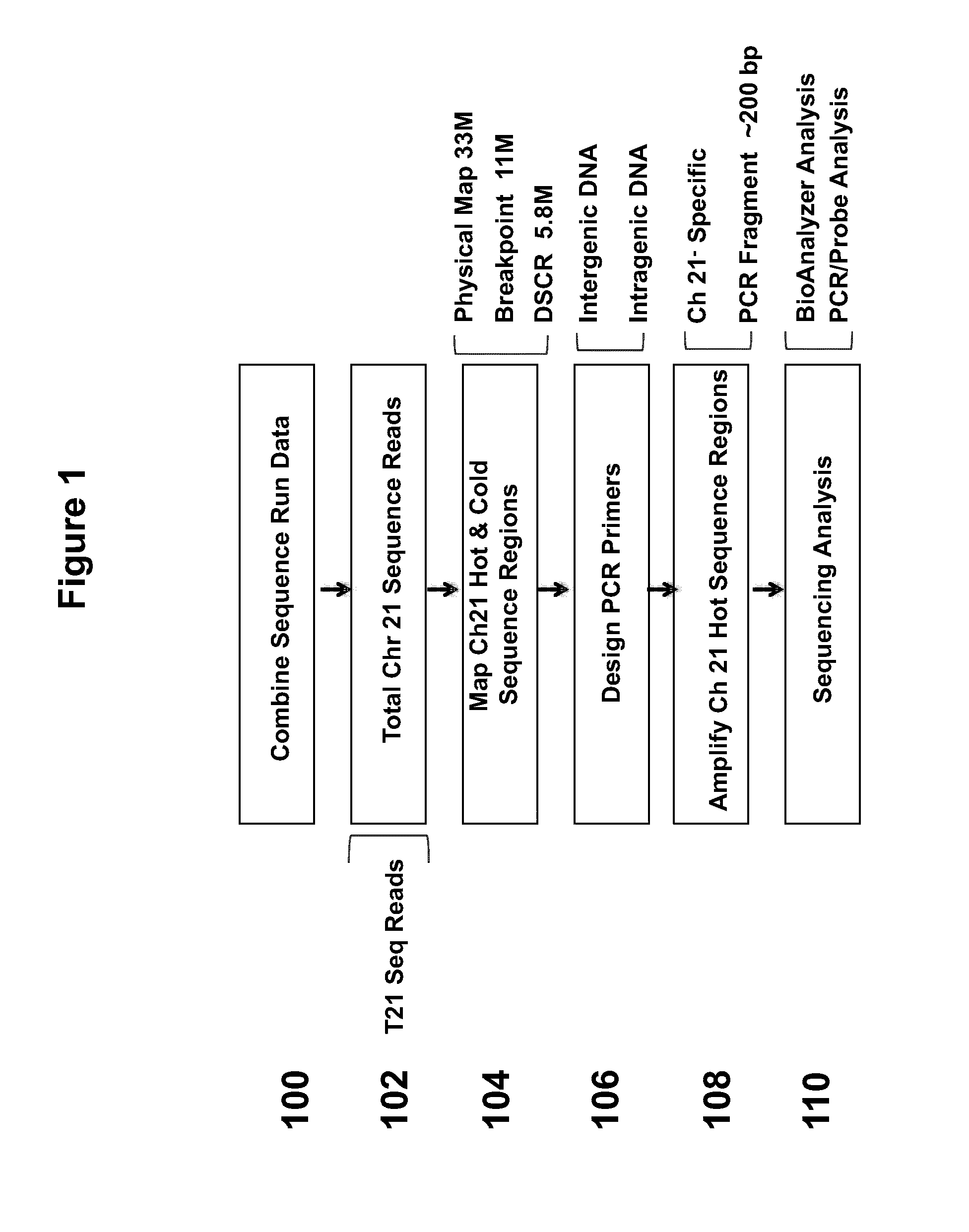
[0099]FIG. 1 illustrates a strategy for selecting sequences from chromosome 21 for enrichment. In step 100, sequence run data was combined. Total chromosome 21 sequence reads were used (102). These samples can include reads from samples that contain trisomy 21. “Hot” and “cold” regions of sequence coverage were mapped on chromosome 21 (104). For example, the region examined can be within a 5.8 Mb Down syndrome critical region (DSCR). PCR primers are designed, which can anneal to intergenic DNA or intragenic DNA (106). The primers were designed to anneal specifically with chromosome 21. The regions to be amplified can be a hot spot region, or region to which a number of sequence reads map (108). The PCR fragments generated can be approximately 200 bp in length. Next, sequencing analysis is performed using BioAnalyzer analysis and / or PCR / probe analysis (110).
[0100]PCR primers were designed to generate amplicons of approximately 200 bp and 150 bp from c...
example 2
Chromosome Walk Strategy for Sequence Enrichment
[0108]FIG. 11 illustrates an overview of the chromosome walk strategy for sequence enrichment. A 5.8 Mbp Down syndrome critical region was selected (1100). PRIMER-BLAST (1102) was used to design 100 PCR primers (1104) in 50,000 bp regions. Unique sequences on chromosome 21 were sought to generate approximately 140-150 bp fragments. Primers were selected from different clusters in different regions on chromosome 21 (1106) and synthesized and arranged in 96 well plates (1108).
[0109]FIG. 12 illustrates a primer pair that was designed, indicating length, annealing position on chromosome 21, melting temperature (Tm), and percent GC content. FIG. 13 illustrates the positions of three 50 kbp regions in a Down syndrome critical region on chromosome 21. FIG. 14 illustrates Bioanalyzer results of PCR amplification of different sequences from clusters A, B, and C in regions A, B, and C on chromosome 21. FIG. 15 illustrates amplification results f...
example 3
Selection of Hotspot Region for Amplification
[0113]Sequences for enrichment can be chosen on the basis of being in a “hotspot,” a region of relatively high sequence coverage. FIG. 19 illustrates that sequence runs from multiple samples were combined to give 79% coverage of chromosome 21. The bottom chart illustrates Illumina pipeline output files containing multiple files and each given start and end chromosome positions; therefore the sequencing reads cover 37 M region (46,927,127 last position-9,757,475 1st position=˜37 M). FIG. 20 shows a schematic of chromosome 21 to which sequence reads have been mapped. Some regions have more sequence coverage than other regions. FIG. 21 illustrates an example of a process that was used to select a specific region of 251 base pairs for amplification. Sequence within 13,296,000-46,944,323 (illustrated in FIG. 20) was selected for amplification. FIGS. 22A and B illustrate the relative position for a Down syndrome critical region (35,892,000-41,7...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thermal profile | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com