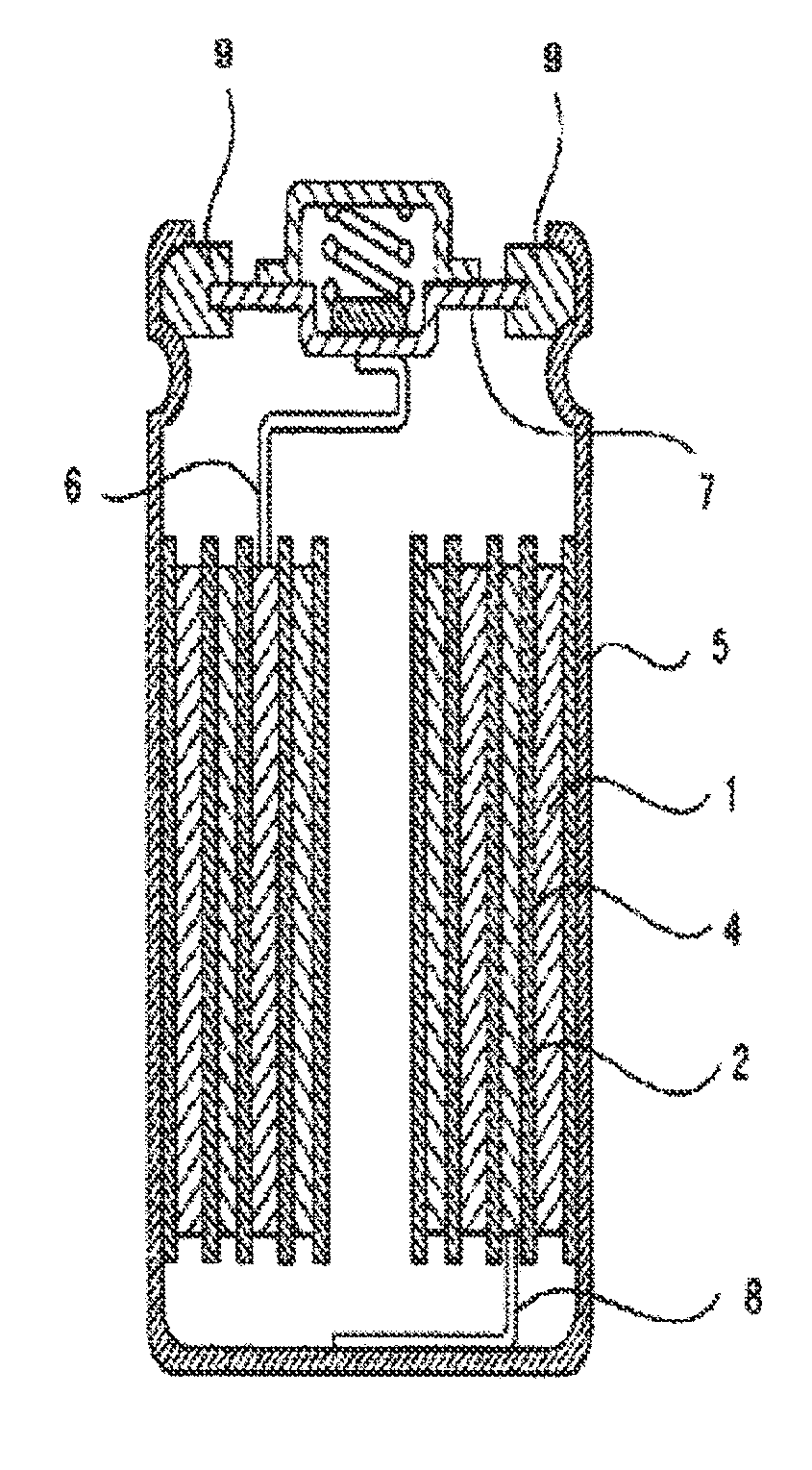
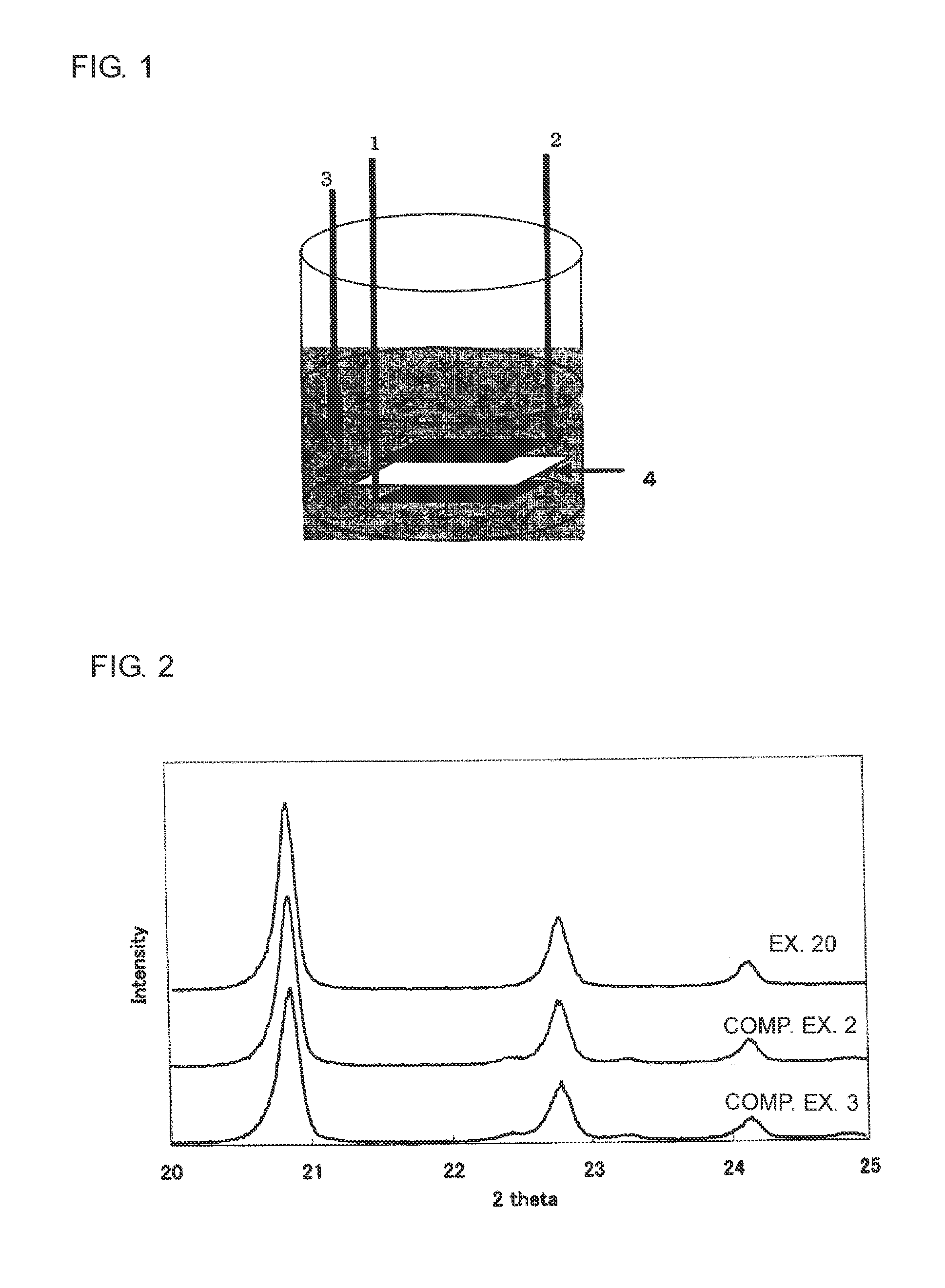
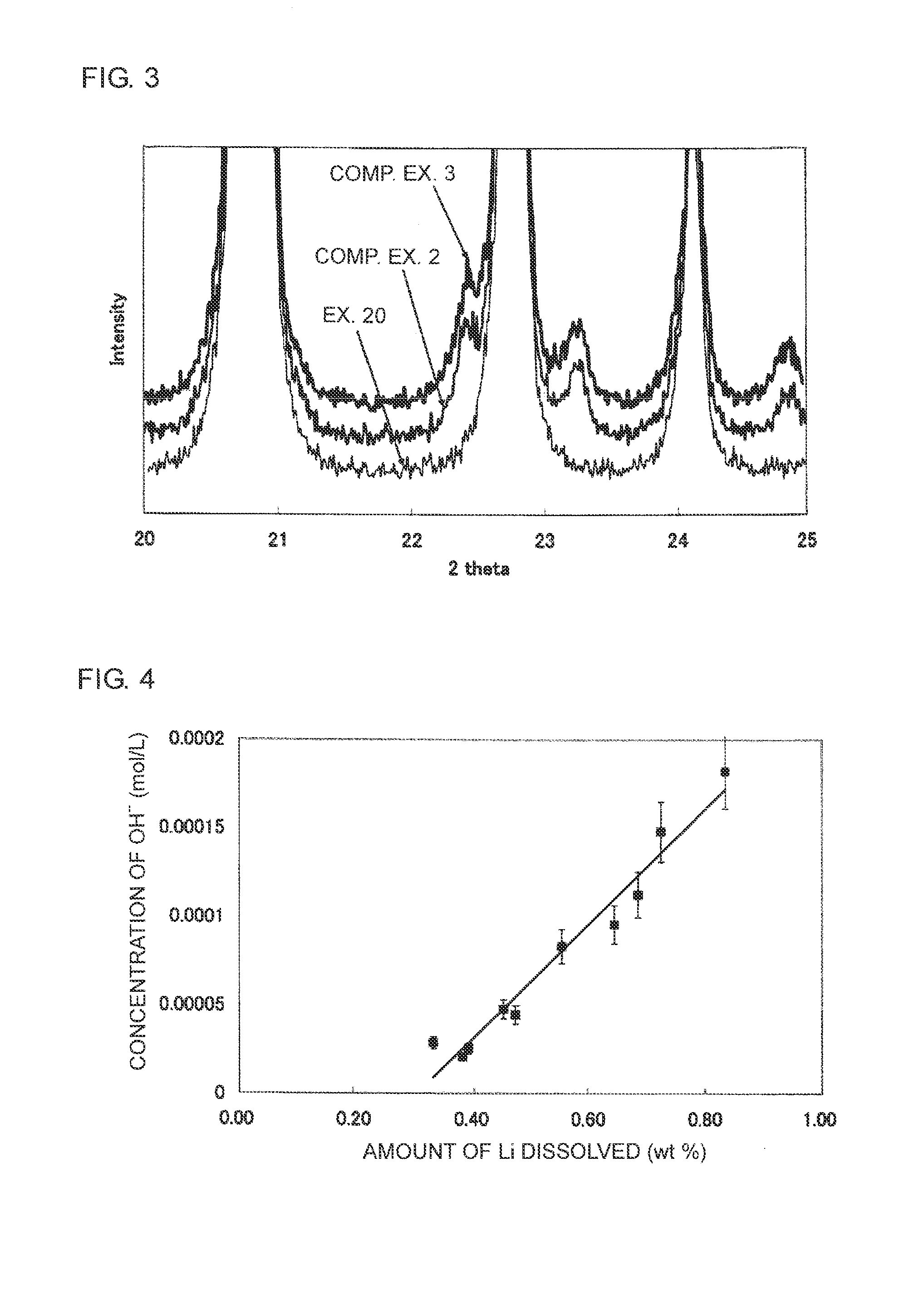
Method of producing an electrode for a lithium secondary battery, and method of producing a lithium secondary battery
a secondary battery and lithium-ion battery technology, applied in the direction of cell components, final product manufacturing, sustainable manufacturing/processing, etc., can solve the problems of large quantity of lithium ions dissolving in water, low sensitivity and precision, and inability to eliminate water-soluble impurities such as lisub>2/sub>cosub>3 /sub>cannot be eliminated, etc., to achieve high sensitivity and precision, and analyze accurately
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment
Example 1
Preparation of Cleaning Solution
[0044]A pH standard solution of a phthalic salt (pH 4.01) produced by KISHIDA CHEMICAL Co., Ltd. was used as a cleaning solution. The cleaning solution at this time was at pH 4.0.
[0045]100 mg of a LiFePO4 sample (sample A) including Li3PO4 was weighed out and 10 ml of the cleaning solution was added to this sample, and the resulting mixture was cleaned for 1 hour by ultrasonic treatment in an ultrasonic pretreating apparatus.
[0046]The cleaning solution after the above cleaning was filtrated with a filter in order to remove the sample not dissolved by cleaning, and an amount of P dissolved in the cleaning solution was quantified by Inductively Coupled high frequency Plasma emission spectroscopic analysis (ICP emission spectroscopic analysis).
[0047]The amount of P dissolved was calculated by the following equation.
Amount of P dissolved (% by weight)=(amount of P dissolved in a cleaning solution (mg)×100) / amount of sample (mg)
[0048]In order to i...
example 2
[0049]Acetic acid and sodium acetate were mixed in a ratio of 1:1 by weight and pure water was added to the resulting mixture to prepare a 1.0% by weight aqueous solution of this mixture, and this aqueous solution was used as a cleaning solution. The cleaning solution at this time was at pH 4.5. A sample was cleaned, the amount of P dissolved in the cleaning solution was quantified, and a pH value of the cleaning solution was measured by the same procedure as in Example 1 except for using this cleaning solution.
example 3
[0050]Acetic acid and sodium acetate were mixed in a ratio of 1:10 by weight and pure water was added to the resulting mixture to prepare a 1.0% by weight aqueous solution of this mixture, and this aqueous solution was used as a cleaning solution. The cleaning solution at this time was at pH 5.6. A sample was cleaned, the amount of P dissolved in the cleaning solution was quantified, and a pH value of the cleaning solution was measured by the same procedure as in Example 1 except for using this cleaning solution.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com