Smac mimetec
a technology of mimetics and smac, applied in the field of smac mimetics, can solve the problems of increasing the apoptosis of abnormally proliferating cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis
[0041]
4-(tert-Butyl-dimethyl-silanyloxy)-pyrrolidine-1,2-dicarboxylic acid 1-benzyl ester (2): A solution of Z-Hyp-OH (1, 300 g, 1.13 mol), TEA (395 mL, 2.83 mol), and DBU (17.2 g, 1.13 mol) in DMF (1.25 L) was stirred in a cold water bath while a suspension of TBS-Cl (188 g, 1.24 mol) in DMF (270 mL) was added slowly at 21-26° C. [Note: moderately exothermic]. The resulting thin suspension was stirred for 22 h at ambient temperature. The reaction mixture was cooled to 2° C. and quenched with water (1.54 L) at ≦26°C. [Note: the pH of the aqueous layer was 8.5-9.0]. MTBE (3 L) was added and the mixture was acidified to pH 3-4 with conc. HCl (168 g) at 17-19° C. The organic layer was separated and washed with water (2×1.5 L). The organic layer was concentrated in vacuo and dried by additional MTBE distillation. Toluene (2×500 mL) was added and distilled to remove moisture to provide 603 g of 2 as a light yellow-colored oil [Note: the water content by KF analysis was 508 ppm]....
examples 2 , 3 , 4
Examples 2, 3, 4, and 5
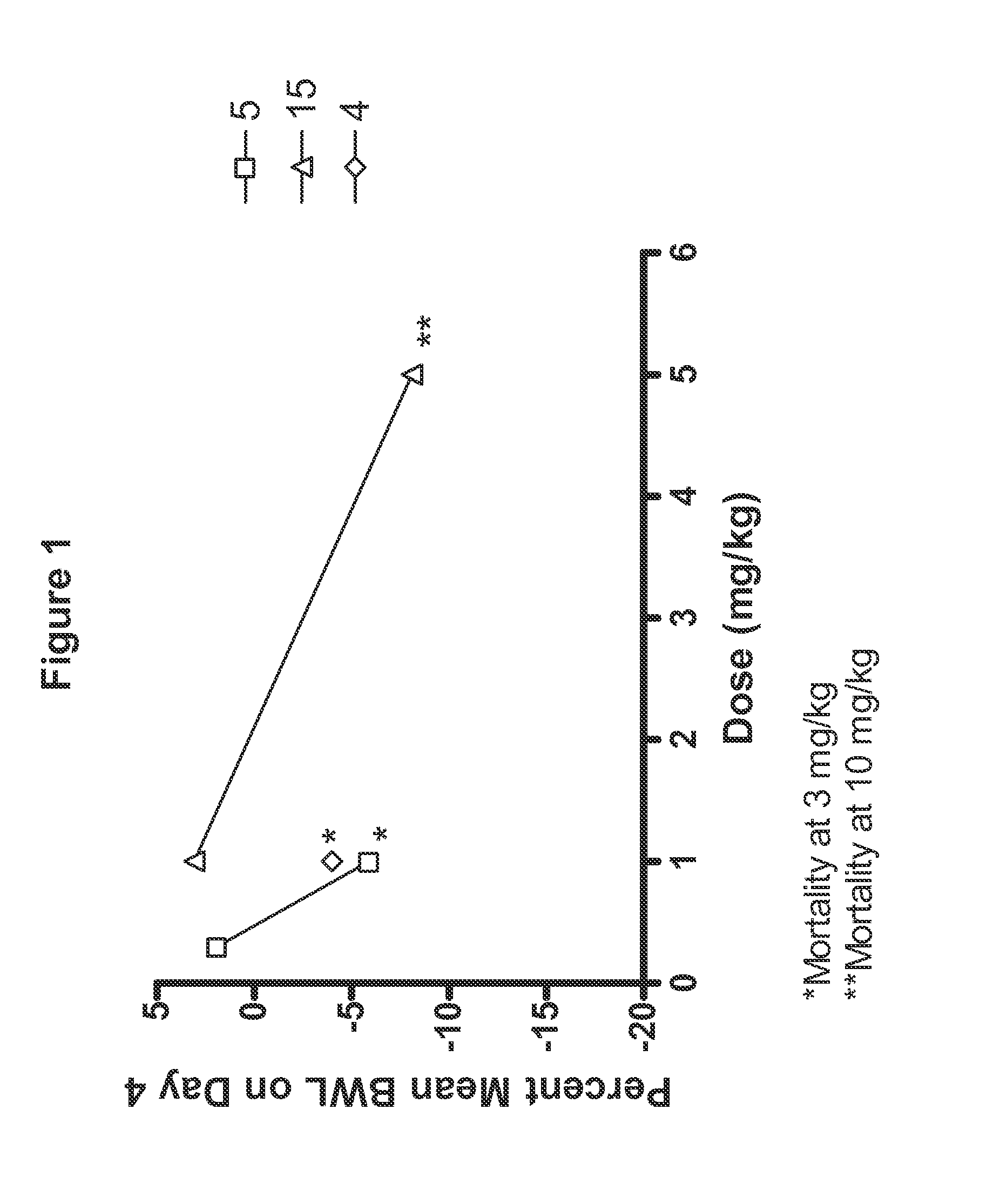
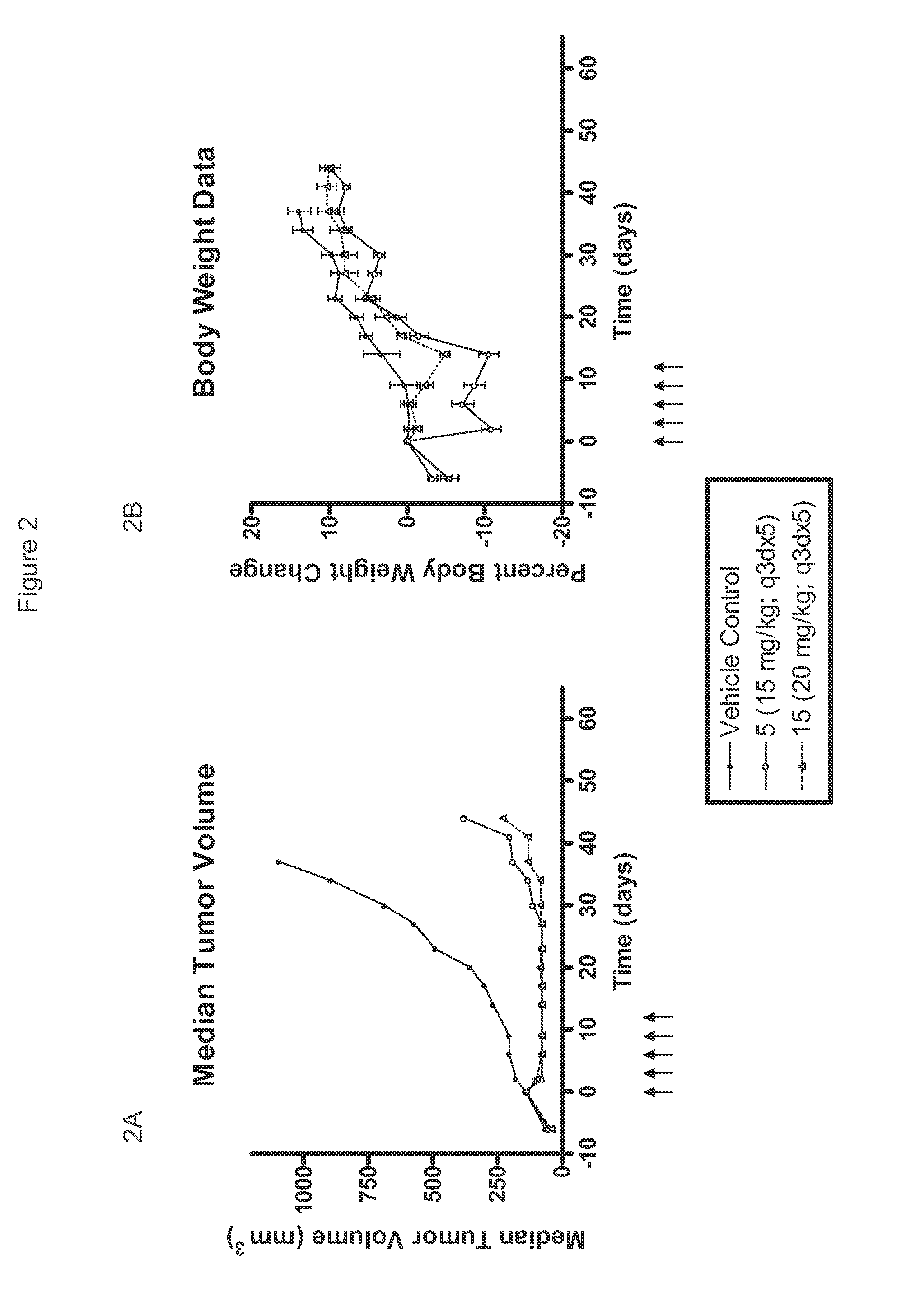
[0053]Compounds tested in Examples 2, 3, 4, and 5 are shown in Table 1.
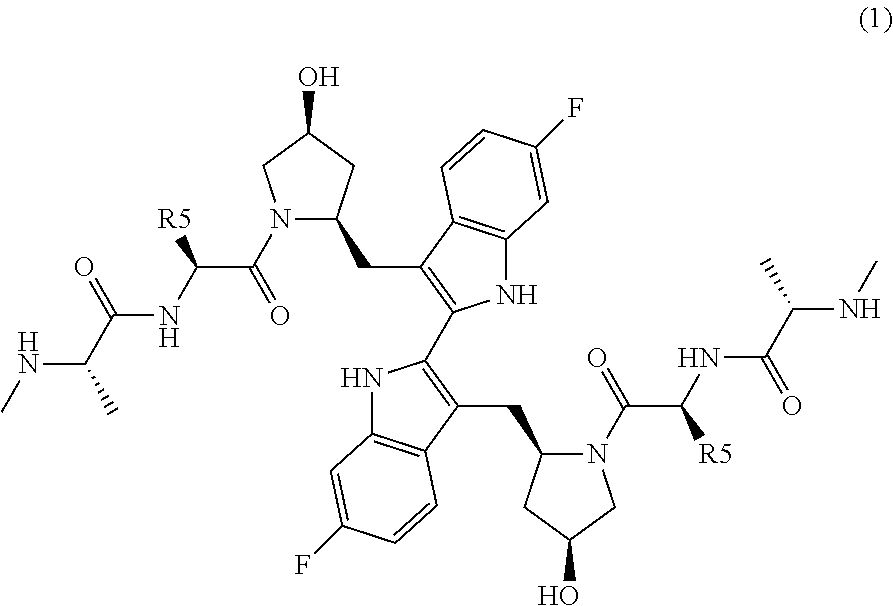
TABLE 1CompoundR5R15—CH2CH36-F 2—CH(CH3)CH36-F 3—R—CH(OH)CH36-F 4—S—CH(OH)CH36-F 5—R—CH(OCH3)CH36-F
example 2a
cIAP Degradation Assay
[0054]The concentration inducing degradation of cIAP-1 and cIAP-2 by 50% (IC50) for various compounds was determined by monitoring the disappearance of Green Fluorescent Protein (GFP)-signal in A375 cells. Briefly, A375 cell lines expressing GFP-tagged cIAP-1 and cIAP-2 were generated by transfecting HA2xEGFP-pcDNA3 vector containing either cIAP-1 (A375Gc1) or cIAP-2 (A375Gc2) coding region. 2×104 of A375Gc1 or A375Gc2 cells were grown in 96-well plate and treated with various concentrations of test compounds for 2 h. After incubation, cells were collected by trypsinization and suspended in 150 μl of DMEM-10% FBS. A total of 104 cells were analyzed using a FACScan (Becton Dickinson). GFP fluorescence was monitored by using an excitation filter at 488 nm and emission was measured with a 530 nm filter. IC50 is defined as the concentration of drug at which 50% of GFP signal was inhibited. Results of the cIAP-1 and -2 degradation assay are shown in Table 2.
TABLE 2G...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com