Gfp fusion proteins and their use
a technology of fusion proteins and fusion proteins, applied in the field of gfp fusion proteins and their, can solve the problems of difficult to prove and not give results reflective of the natural sta
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
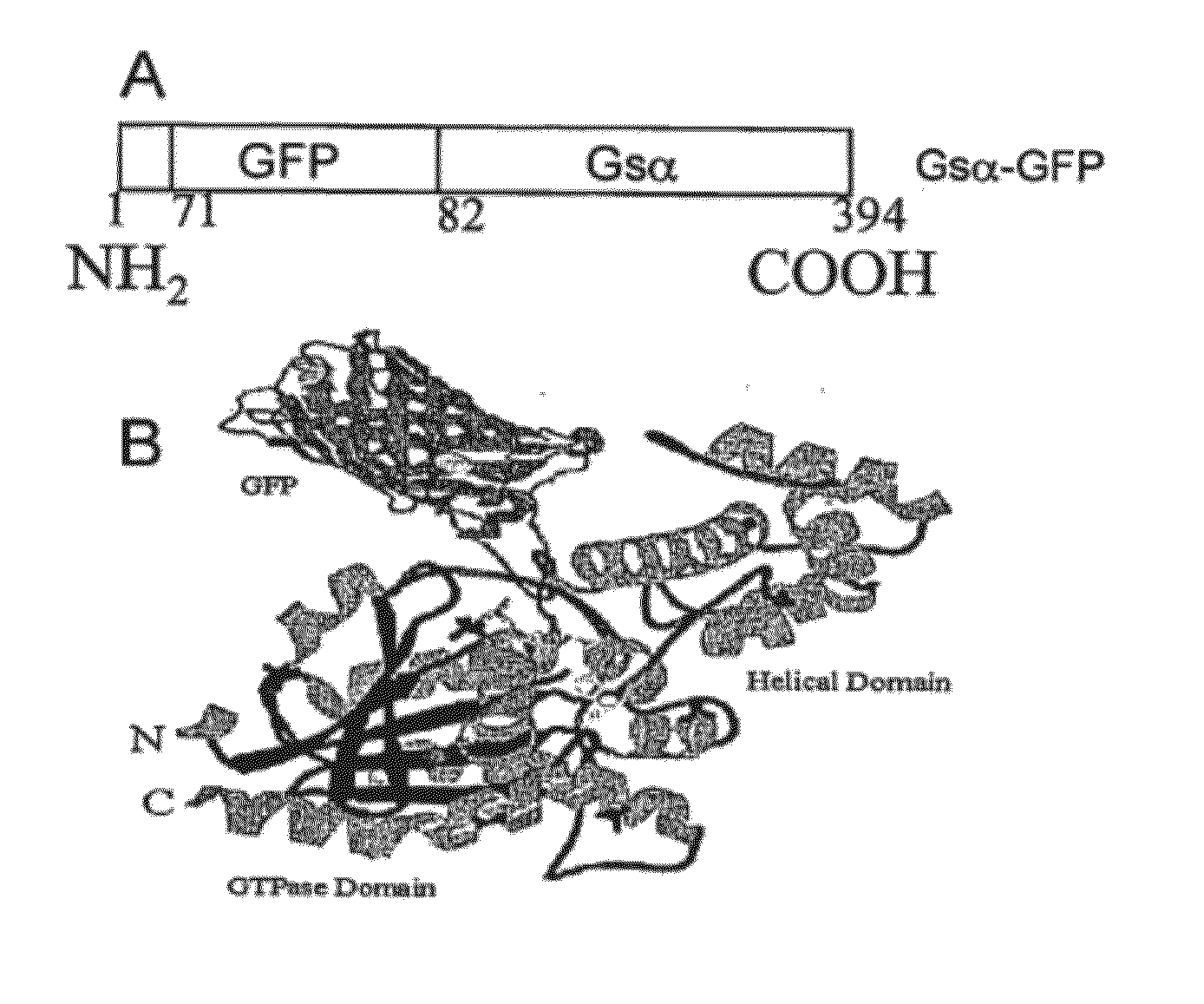
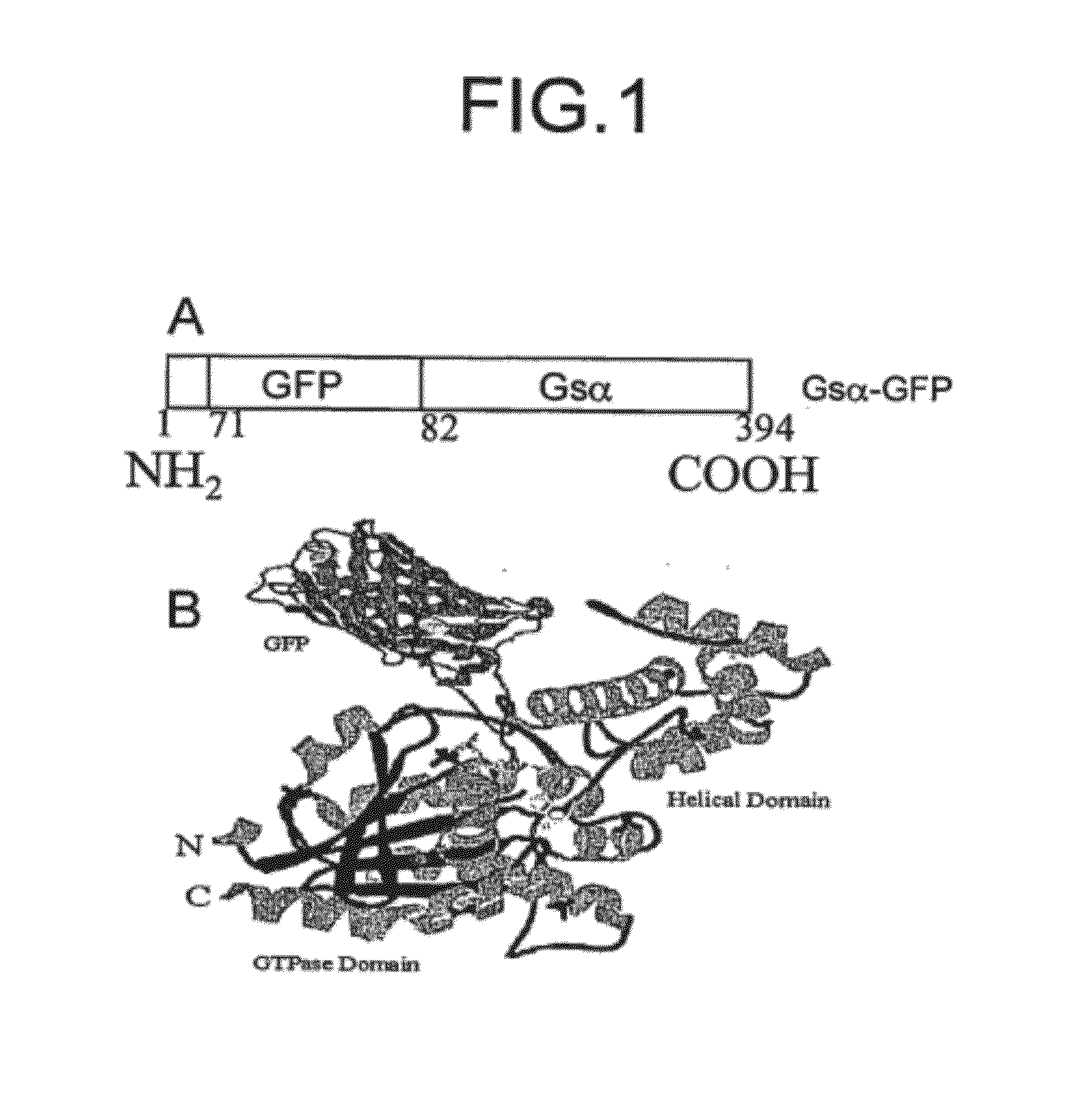
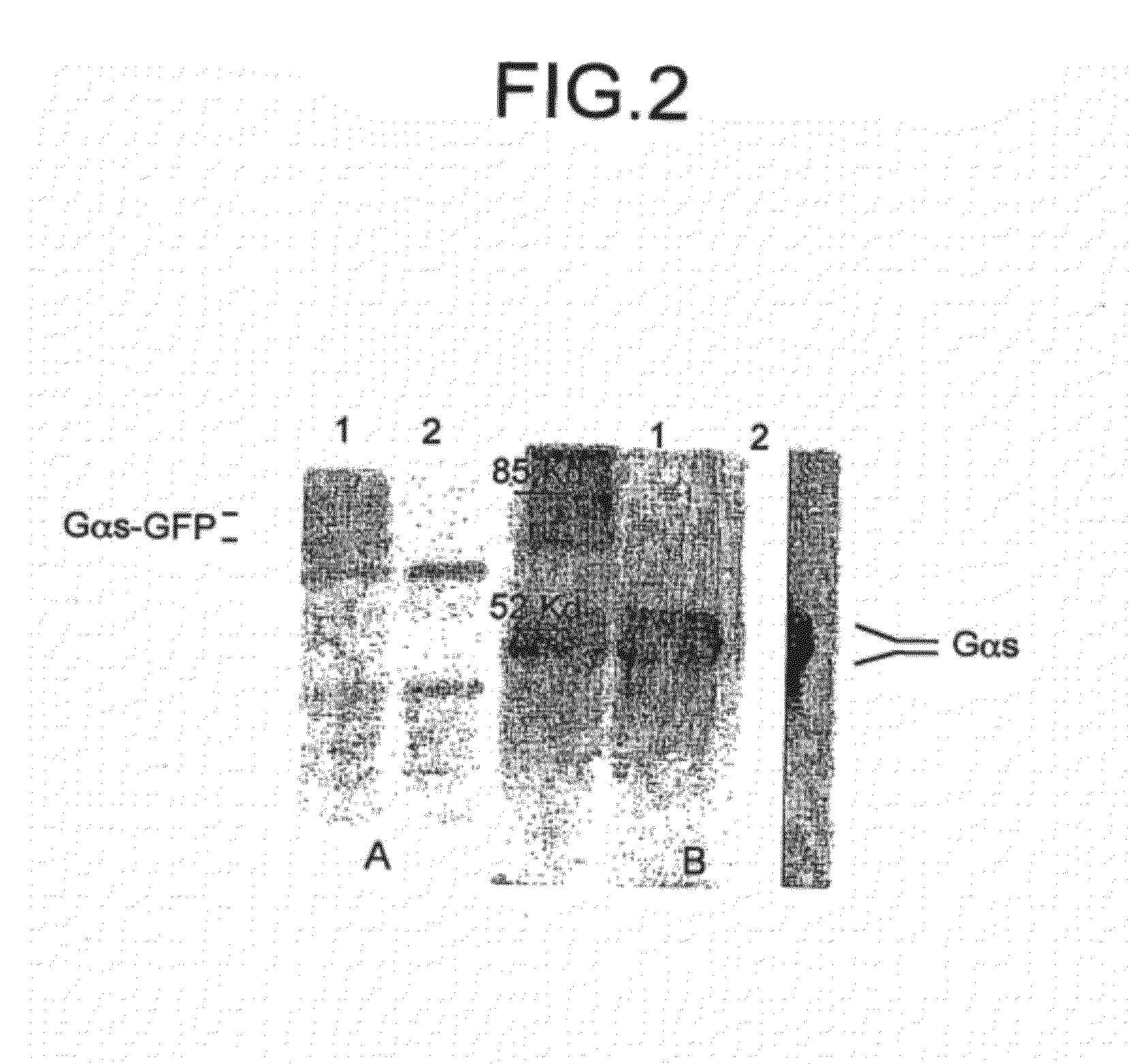
[0029]Construction of Gαs-GFP
[0030]Full length cDNAs encoding Gαs were excised from the PcDNA-1 vector by digesting with Sam I and Xba I restriction enzymes. The full length EGFP cDNA was obtained by PCR from the PEGFP-N3 using appropriate primers (sense 5′ GGAATTCATGAGCAAGGGCGAGGAACTG-3′ (SEQ ID NO: 8); antisense 5′-GCTCTAGACGACTTGTACAGCTCGT-3′) (SEQ ID NO: 9) and adding restriction sites to its cDNA (EcoR I at the initiation codon and Xba I at end of cDNA). To insert the EGFP within the sequence of Gαs, the first fragment of Gαs (from 1 to 71 amino acids) was amplified by PCR with restriction sites for Kap 1 at initiation codon and EcoR I at end of the fragment. The cDNA of the fragment was cloned into PcDNA3 vector by the Kap 1 and EcoR 1 restriction sites using primers (sense 5′GGGTACCATGGGCTGCCTCGGCAACA-3′ (SEQ ID NO: 10); antisense 5′-GGAATTCGTCCTCTTCGCCGCCCTTCT-3′) (SEQ ID NO: 11). Modified 7 EGFP cDNA was spliced into the first fragment of Gαs by EcoR 1 and Xba 1 restriction...
PUM
| Property | Measurement | Unit |
|---|---|---|
| total volume | aaaaa | aaaaa |
| total volume | aaaaa | aaaaa |
| total volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com