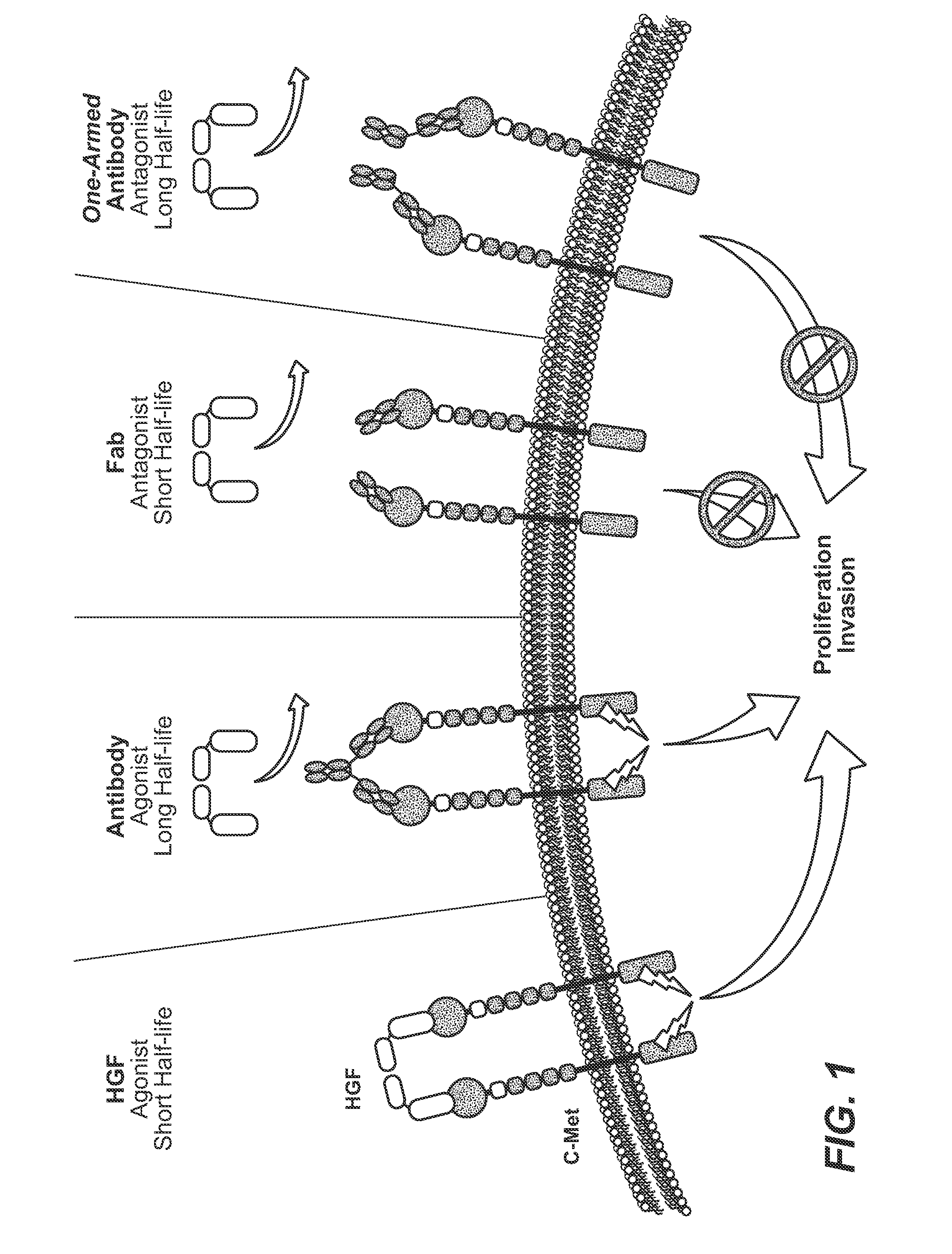
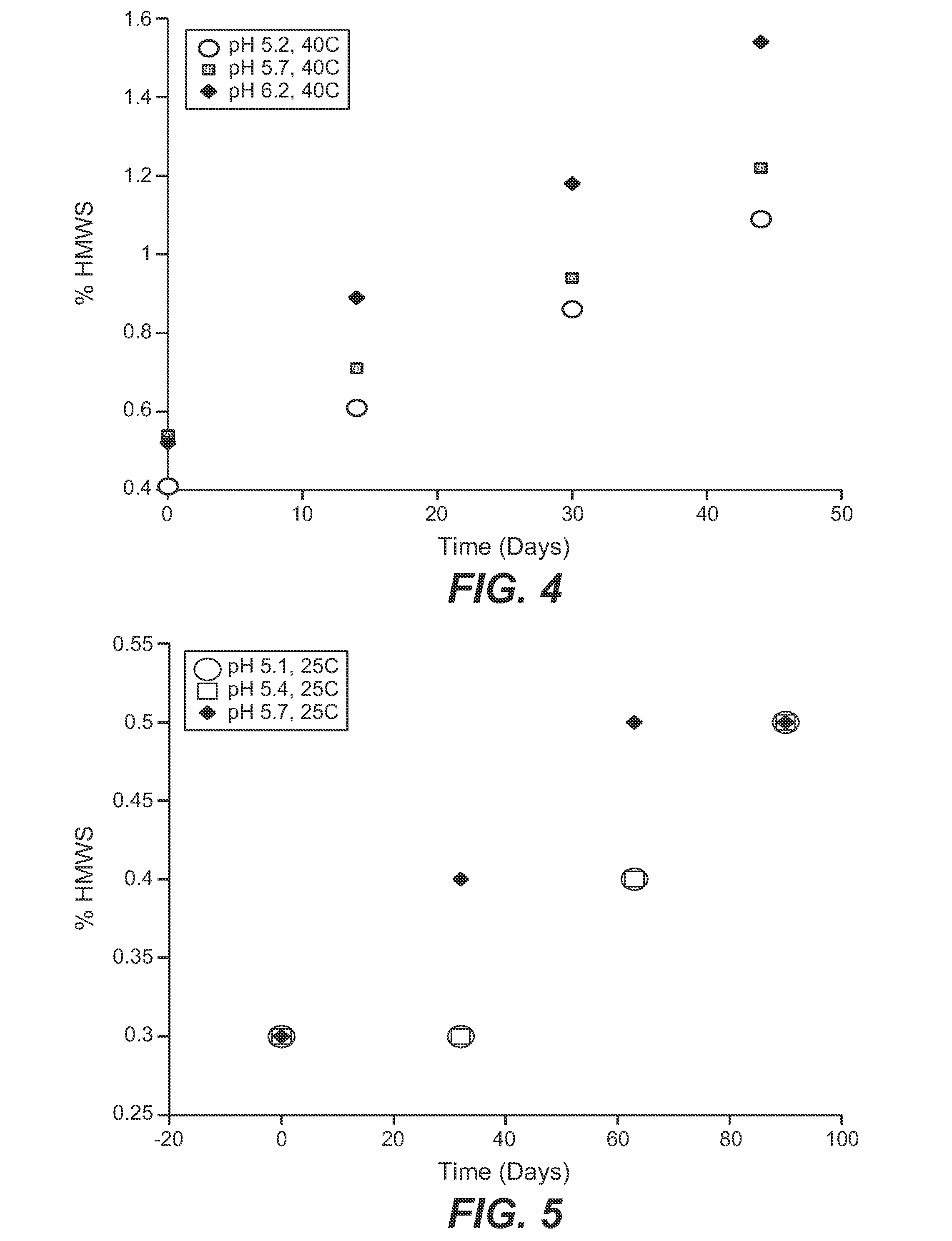
Anti-c-met antibody formulations
an anticmet and antibody technology, applied in the field of anticmet antibody formulations, can solve the problems of loss of stabilization mechanism, large polypeptides, special problems, etc., and achieve the effects of increasing the complexity of polypeptides, reducing the stability of antibodies, and increasing the stability of antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
2. The pharmaceutical formulation of embodiment 1, wherein the anti-c-met antibody comprises a HVR-L1 comprising sequence KSSQSLLYTSSQKNYLA (SEQ ID NO:1), a HVR-L2 comprising sequence WASTRES (SEQ ID NO:2), a HVR-L3 comprising sequence QQYYAYPWT (SEQ ID NO:3), a HVR-H1 comprising sequence GYTFTSYWLH (SEQ ID NO:4), a HVR-H2 comprising sequence GMIDPSNSDTRFNPNFKD (SEQ ID NO:5), and a HVR-H3 comprising sequence ATYRSYVTPLDY (SEQ ID NO:6).
3. The pharmaceutical formulation of any one of embodiments 1-2, wherein the anti-c-met antibody comprises (a) a heavy chain variable domain comprising the sequence: EVQLVESGGGLVQPGGSLRLSCAASGYTFTSYWLHWVRQAPGKGLEWVGMIDPSNSDT RFNPNFKDRFTISADTSKNTAYLQMNSLRAEDTAVYYCATYRSYVTPLDYWGQGTLVTV SS (SEQ ID NO:19) and (b) a light chain variable domain comprising the sequence: DIQMTQSPSSLSASVGDRVTITCKSSQSLLYTSSQKNYLAWYQQKPGKAPKLLIYWASTR ESGVPSRFSGSGSGTDFTLTISSLQPEDFATYYCQQYYAYPWTFGQGTKVEIKR (SEQ ID NO:20).
4. The pharmaceutical formulation of any one of embodiments 1...
embodiment 4
5. The pharmaceutical formulation of embodiment 4, wherein the first and second Fc polypeptides form a Fc region that increases stability of said antibody fragment compared to a Fab molecule comprising said antigen binding arm.
6. The pharmaceutical formulation of any one of embodiment 1-5, wherein the anti-c-met antibody comprises (a) a first polypeptide comprising the amino acid sequence of SEQ ID NO:19, a CH1 sequence, and a first Fc polypeptide and (b) a second polypeptide comprising the amino acid sequence of SEQ ID NO:20 and CL1 sequence.
embodiment 6
7. The pharmaceutical formulation of embodiment 6, wherein the anti-c-met antibody further comprises (c) a third polypeptide comprising a second Fc polypeptide.
8. The pharmaceutical formulation of any one of embodiments 1-7, wherein the first Fc polypeptide comprises the Fc sequence depicted in FIG. 2 (SEQ ID NO: 17) and the second Fc polypeptide comprises the Fc sequence depicted in FIG. 3 (SEQ ID NO: 18).
9. The pharmaceutical formulation of any one of embodiments 1-8, wherein the anti-c-met antibody is onartuzumab.
10. The pharmaceutical formulation of any one of embodiments 1-8, wherein the anti-c-met antibody binds the same epitope as onartuzumab.
11. The pharmaceutical formulation of any one of embodiments 1-10, wherein the anti-c-met antibody is present at a concentration between about 10 mg / mL and about 100 mg / mL (e.g. about 15 mg / mL and about 75 mg / mL).
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com