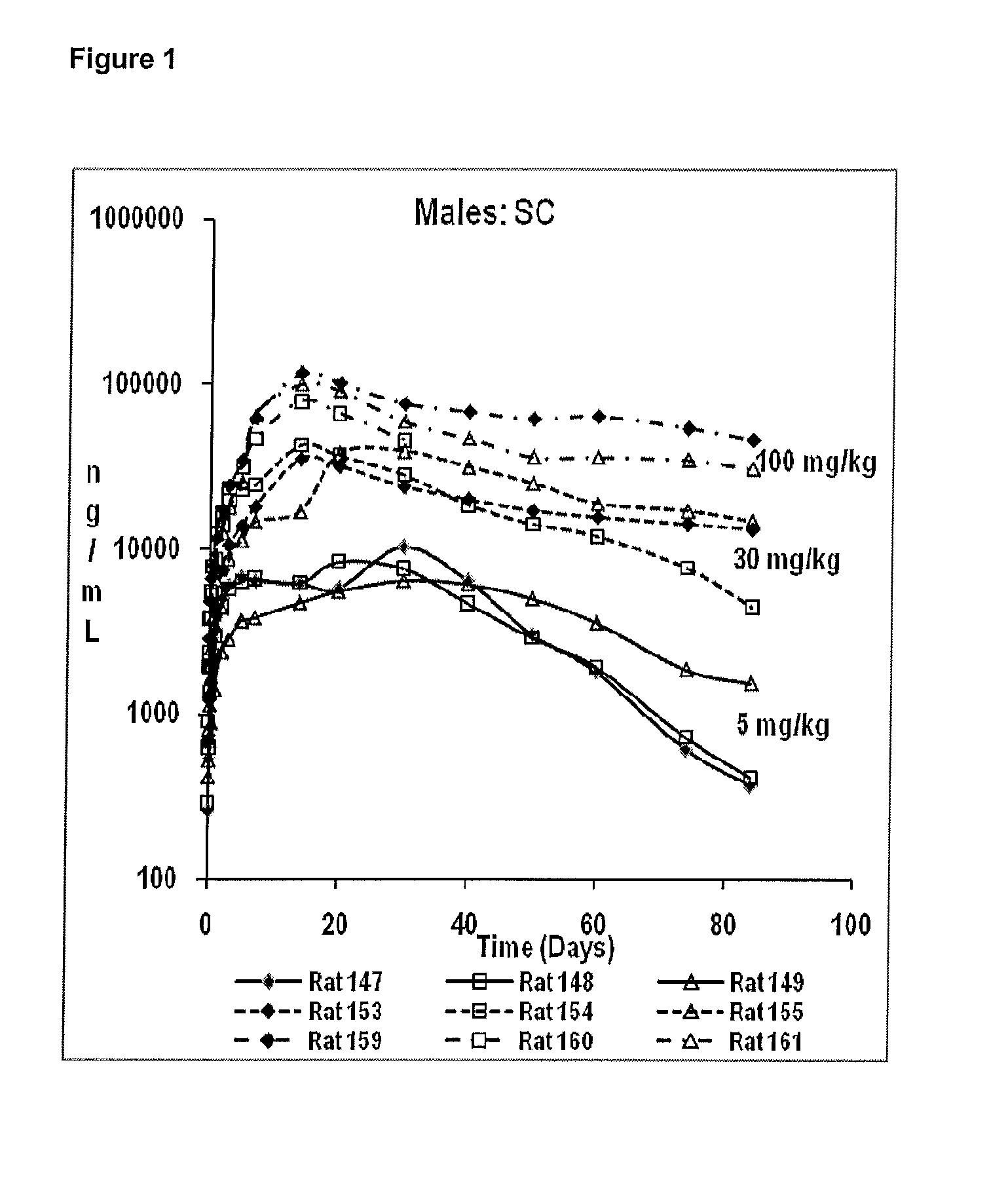
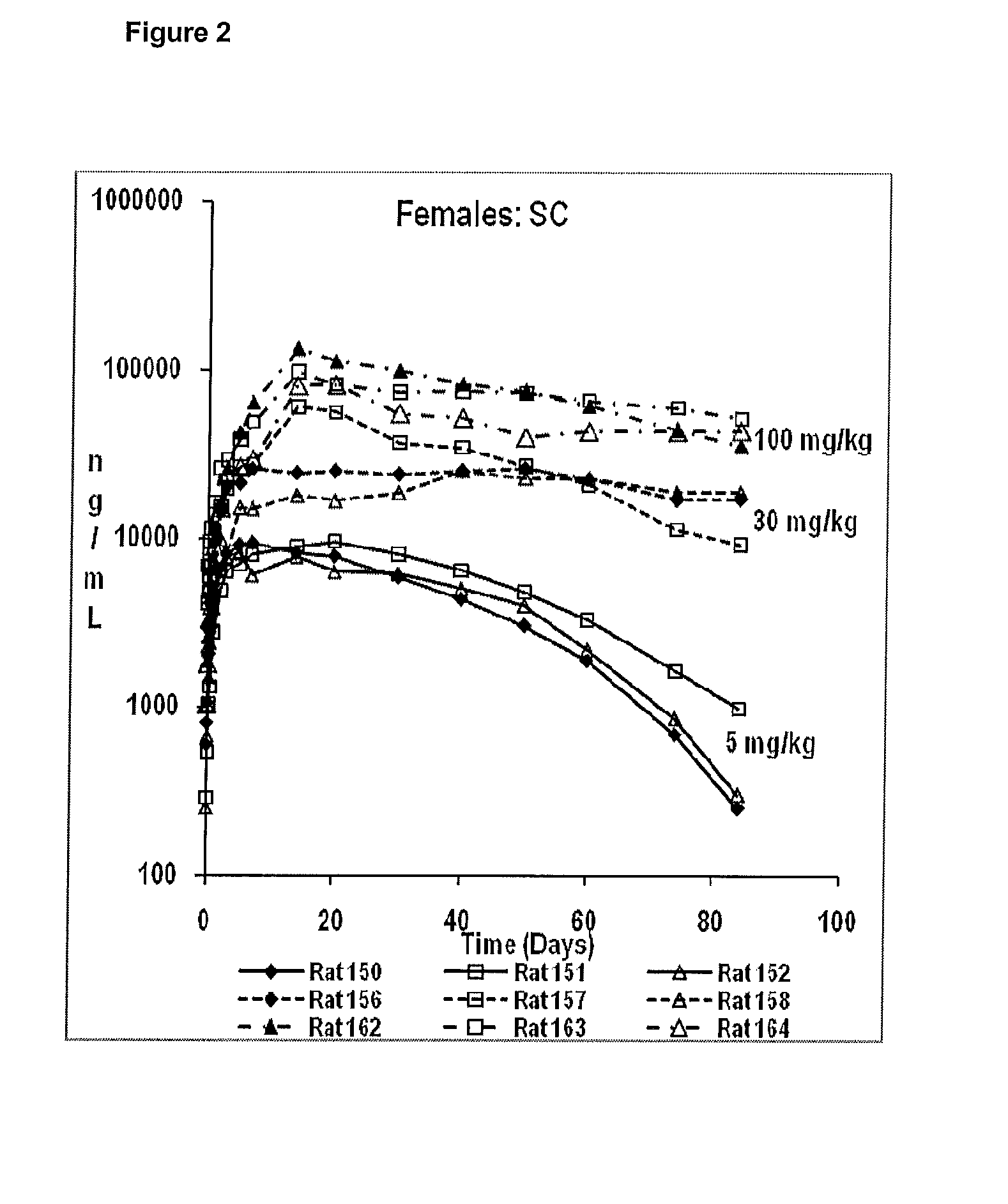
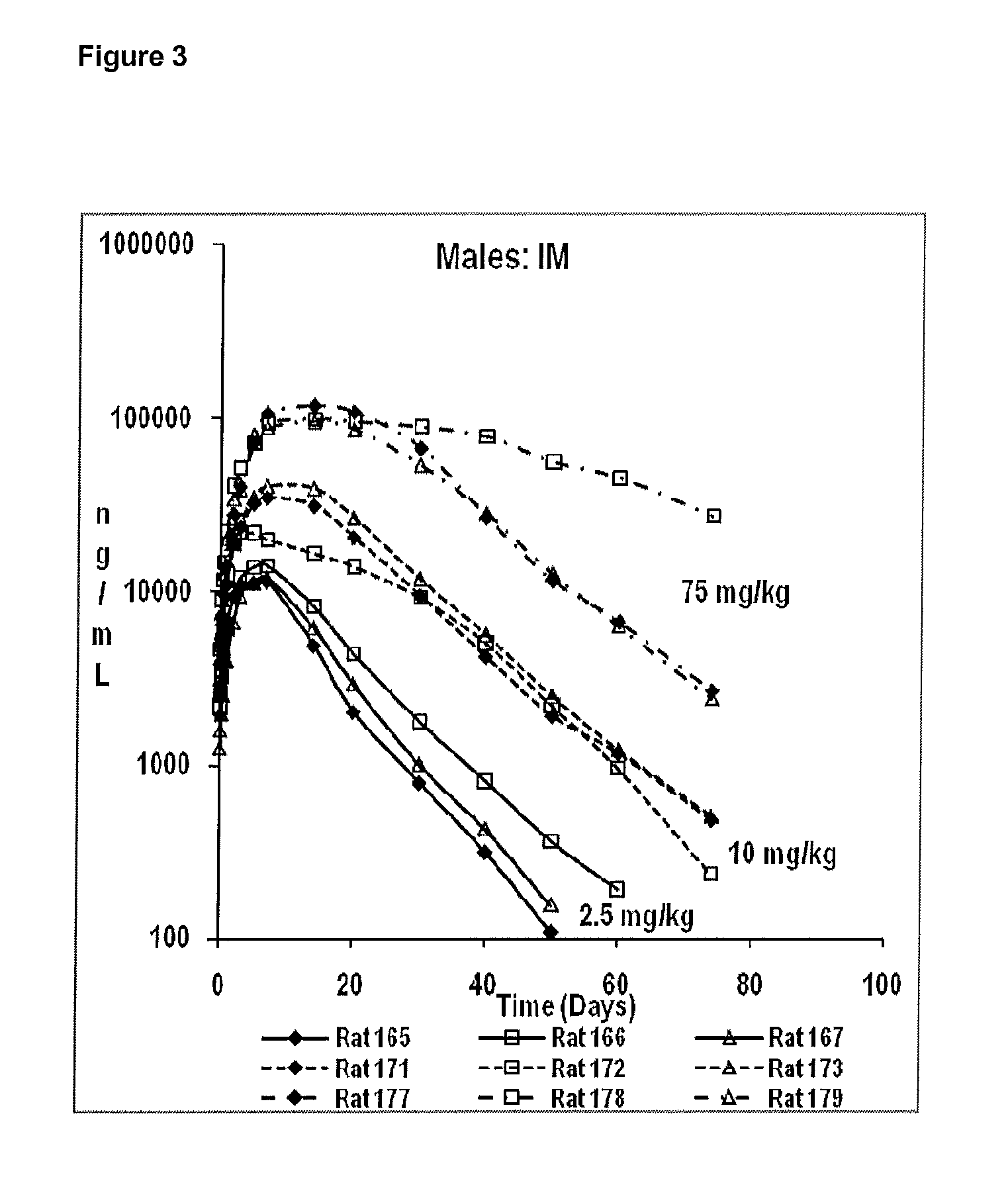
Pharmaceutical Compositions
a technology of compositions and pharmaceuticals, applied in the field of compounded drugs, can solve the problems of multiple drug resistant strains of hiv, patient non-compliance, and well-known patient non-compliance, and achieve the effect of reducing the burden of medication (pill) or difficulty in patient compliance, and maintaining the efficacy of therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Pharmaceutical Composition
[0081]
TABLE 1Composition of a compound of formula (I) Injectable SuspensionQuantityComponent(mg / mL)FunctionCompound of Formula (I)200.0ActiveMannitol45.0Tonicity agentPolysorbate 2020.0Wetting agentPolyethylene Glycol (PEG) 335020.0StabilizerWater for InjectionQS to 1.0 mLSolvent
[0082]Manufacturing Process
[0083]A compound of formula (I), mannitol, polysorbate 20, PEG 3350, and water for injection were compounded and milled using a wet bead mill. The resulting suspension was filled into 3 mL, USP Type I glass vials at a fill volume of 1.5 mL, the vials are stoppered and sealed, and then terminally sterilized by gamma irradiation.
example 2
Particle Size
[0084]A sample of a compound of formula (I) injectable suspension prepared by the process as described in Example 1 was irradiated by gamma irradiation at 29.9-31.5 kGy dose. The milling time was 5 hours. The particle size determined by laser diffraction technique is the following:
[0085]×10=75 nm
[0086]×50=157 nm
[0087]×90=646 nm
[0088]×50 of less than 200 nm was achieved.
example 3
[0089]A sample of a compound of formula (I) injectable suspension prepared by the process as described in Example 1 was irradiated by gamma irradiation at 29.9-31.5 kGy dose. Samples pre gamma irradiation and post gamma irradiation were tested for drug related impurities by HPLC.
Total Drug Related Impurities, % area / areaPre Gamma Irradiation0.19Post Gamma Irradiation0.16
[0090]The formulation is stable upon gamma irradiation.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com