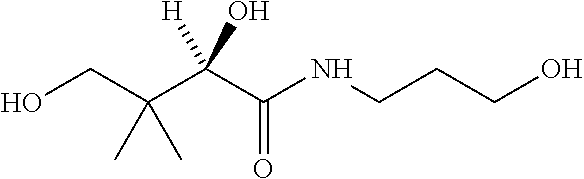
Medical product with a particle-free coating releasing an active substance
a technology of active substances and medical products, applied in the field of medical products, can solve the problems of insolubility in water and particle release during dilatation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of a Coating Solution of Paclitaxel (Ptx) and Dexpanthenol
[0137]Herefore 120 mg paclitaxel (Sigma-Aldrich Chemie GmbH, Germany) is dissolved in acetone. Likewise 30 mg dexpanthenol (Cal Roth GmbH, Germany) is dissolved in 500 μl ethanol. Afterwards both solutions are combined.
example 2a
Preparation of a Coating Solution of Paclitaxel (Ptx) and PEG (1:2:2 w:w:w)
[0138]Herefore 100 mg paclitaxel and 200 mg PEG A and 200 mg PEG B are dissolved in 1000 μl acetone.
[0139]PEG A is PEG 400 (Sigma-Aldrich Chemie GmbH, Germany) and has a molecular mass between 380 and 420 and an average molecular mass Mn of 400.
[0140]PEG B is a polyethylene glycol (Sigma-Aldrich Chemie GmbH, Germany) with a molecular mass between 950 and 1050.
example 2b
Preparation of a Coating Solution of Sirolimus and PEG (1:2:2 w:w:w)
[0141]Herefore 100 mg sirolimus (Merck4Biosciences, Germany) is dissolved in ethyl acetate and 200 mg PEG A and 200 mg PEG B are dissolved in 1000 μl absolute ethanol. Both solutions are combined.
PUM
| Property | Measurement | Unit |
|---|---|---|
| dynamic viscosity | aaaaa | aaaaa |
| dynamic viscosity | aaaaa | aaaaa |
| dynamic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com