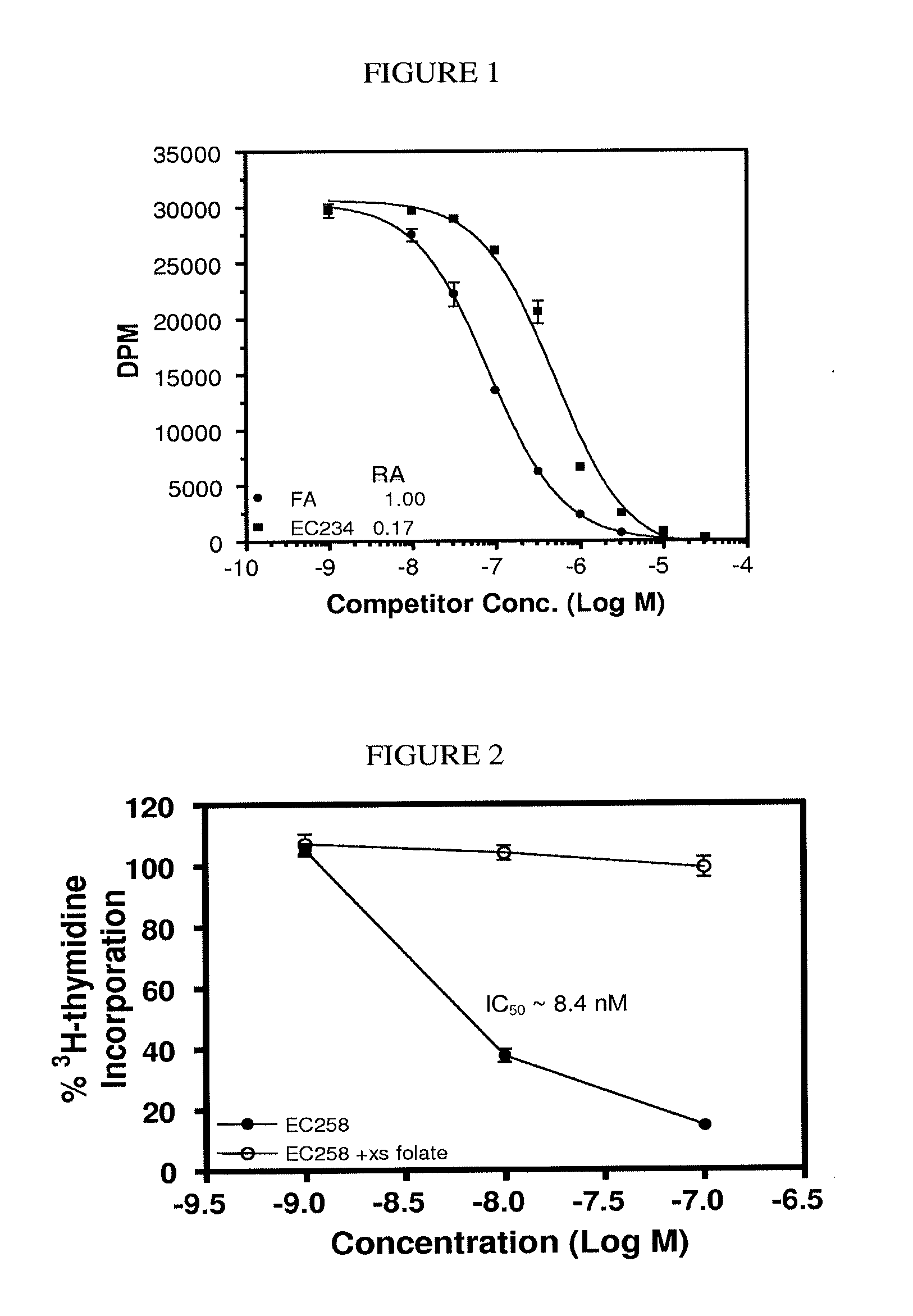
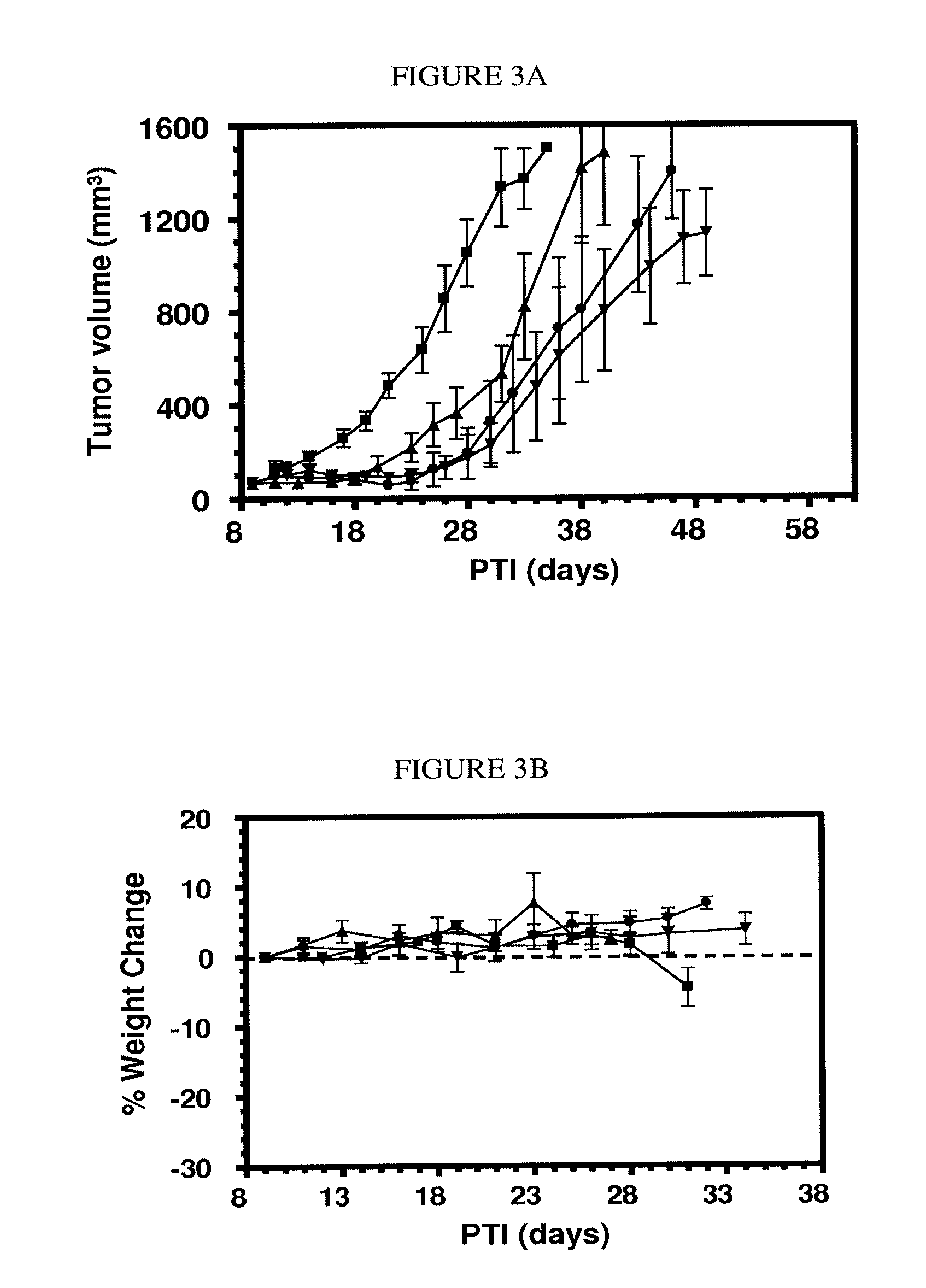
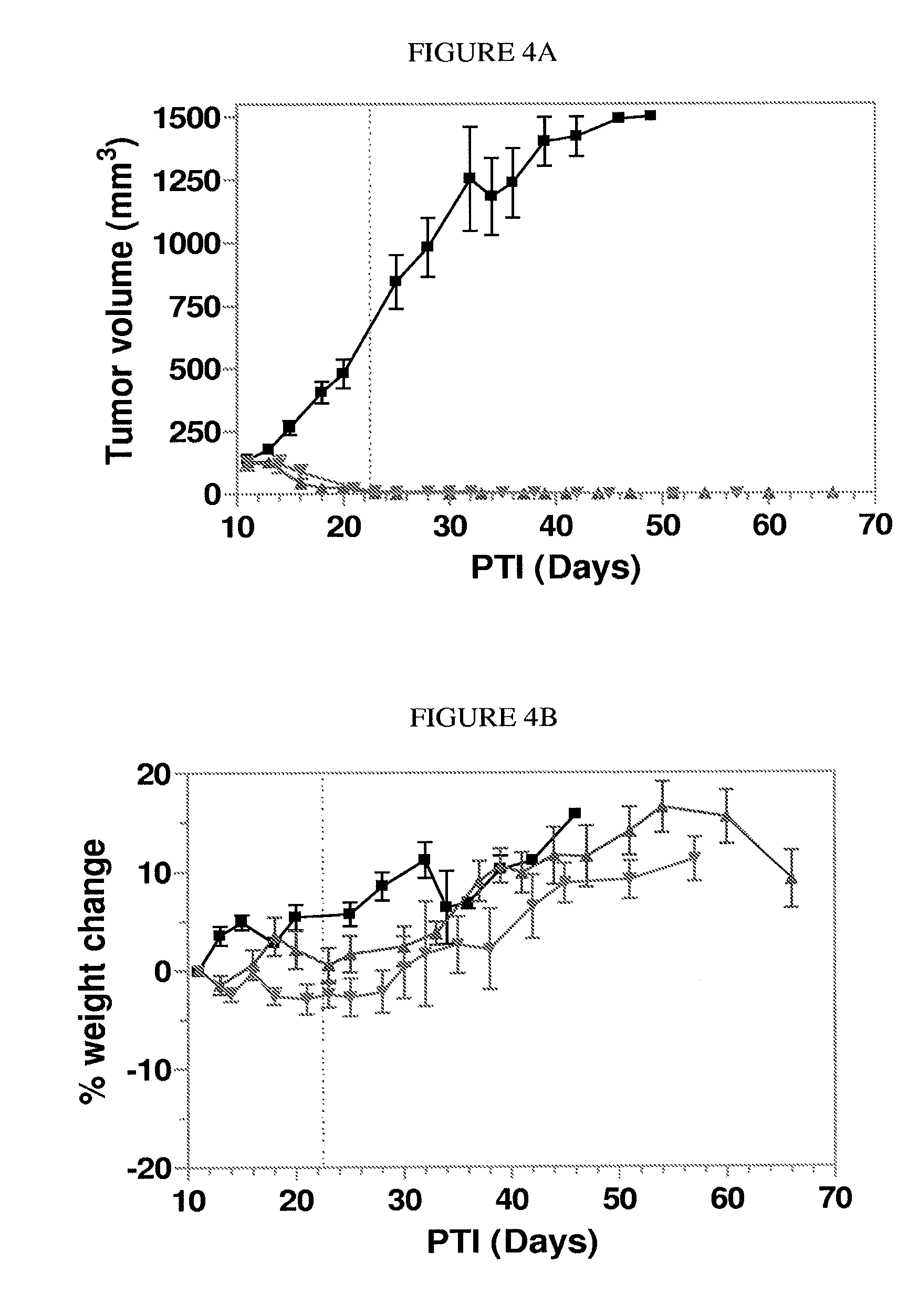
Conjugates containing hydrophilic spacer linkers
a technology of hydrophilic spacer linkers and conjugates, which is applied in the direction of drug compositions, peptides/protein ingredients, antibacterial agents, etc., can solve the problems of complex approaches and adverse side effects of the currently available chemotherapeutic agents and radiation therapy regimens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
[0315]General Method 2 for Preparing Conjugates having a hydrophilic spacer linker (one-pot). Illustrated with preparation of EC0543. DIPEA (7.8 μL) and isobutyl chloroformate (3.1 μL) were added with the help of a syringe in tandem into a solution of tubulysin A (18 mg) in anhydrous EtOAc (0.50 mL) at −15° C. After stirring for 35 minutes at −15° C. under argon, to the reaction mixture was added a solution of EC0311 (5.8 mg) in anhydrous EtOAc (0.50 mL). The cooling was removed and the reaction mixture was stirred under argon for an additional 45 minutes, concentrated, vacuumed, and the residue was dissolved in THF (2.0 mL). Meanwhile, EC0488 (40 mg) was dissolved in deionized water (bubbled with argon for 10 minutes prior to use) and the pH of the aqueous solution was adjusted to 6.9 by saturated NaHCO3. Additional deionized water was added to the EC0488 solution to make a total volume of 2.0 mL and to which was added immediately the THF solution containing the activated tubulysin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| volumes | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com