Vesicle monoamine transporter target bound drug and preparation method thereof
A targeted binding and transporter technology, which is applied in pharmaceutical formulations, preparations for in vivo experiments, radioactive carriers, etc., can solve problems such as unfavorable transportation and long-term storage, high liver background, and affecting the imaging effect of the pancreas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
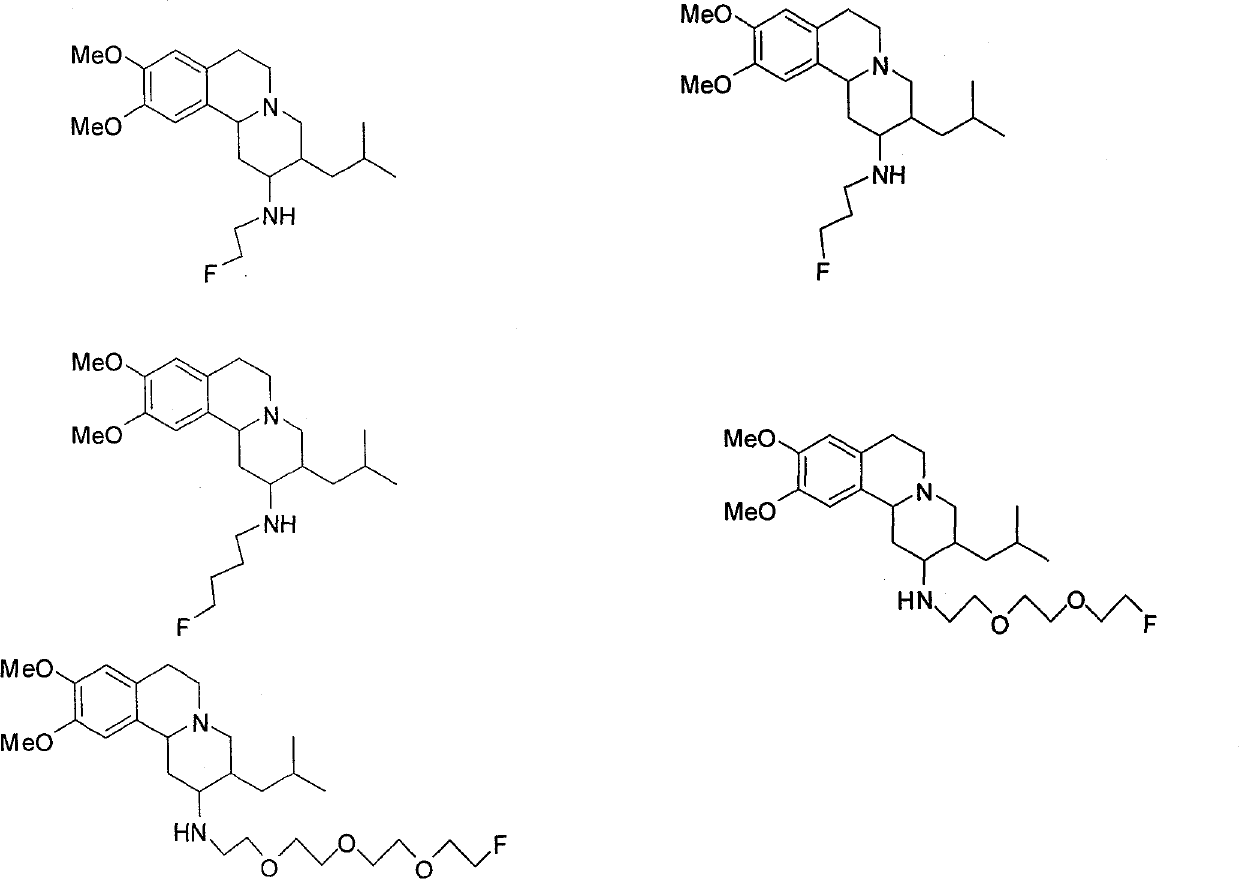
[0038] 1. Synthesis of 2-(2-fluoroethylamino)tetrabenazine (A-1)
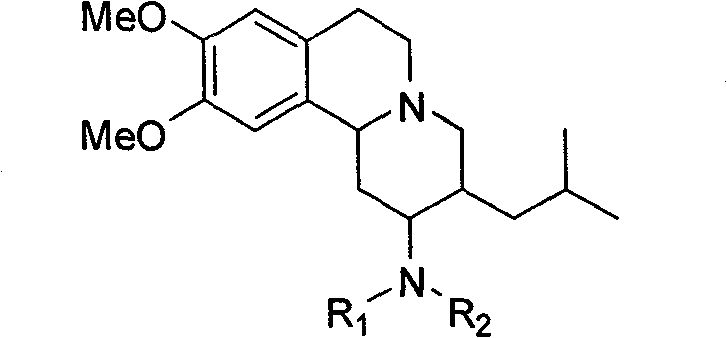
[0039] (1) The structure of the compound
[0040]
[0041] (2) Synthesis method
[0042] Method 1: Add 1mmol tetrabenazine and 5ml absolute ethanol into a two-necked round-bottomed flask, then add 1.5mmol 2-fluoroethylamine hydrochloride and 2mmol triethylamine, under nitrogen protection, heat at 100°C for 12 hours . After cooling to room temperature, 6 mmol of sodium borohydride was added, and the reaction was stirred at room temperature for 6 hours. Stop the reaction, add 50ml of water to dissolve, extract with dichloromethane (3×20ml), combine the organic phases, wash with saturated sodium chloride solution, and add anhydrous sodium sulfate to dry. The solvent was evaporated by filtration and separated by column chromatography. The solvent ratio was ethyl acetate:n-hexane:triethylamine=1:9:0.5. A white solid was obtained with a product yield of 42%.
[0043] Method 2: Dissolve 1mmol 2-aminotetrabenaz...
Embodiment 2
[0049] 1. Synthesis of 2-(3-fluoropropylamino) tetrabenazine (A-2)
[0050] (1) The structure of the compound
[0051]
[0052] (2) Synthesis method
[0053] Take 1mmol of 2-aminotetrabenazine and dissolve it in 5ml of DMF, then add 1.3mol of 2-fluoroethyl p-toluenesulfonate and 1.5mmol of anhydrous potassium carbonate, heat and stir at 80°C for 6 hours to stop the reaction. Add 150ml of ethyl acetate to dilute the reaction solution and wash with H 2 After washing with O (4×15ml) and saturated sodium chloride solution, it was dried by adding anhydrous sodium sulfate. The solvent was evaporated by filtration and separated by column chromatography. The solvent ratio was: ethyl acetate:n-hexane:triethylamine=2:8:0.5. A yellow oily solid was obtained in a yield of 43.6%.
[0054] (3) Confirmation of A-2
[0055] h 1 -NMR (CDCl 3 , 200MHz): δ (ppm) 0.87-0.97 (m, 6H), 1.0-1.34 (m, 4H), 1.37-1.72 (m, 3H), 1.75-2.16 (m, 2H), 2.32-2.8 (m, 6H), 2.88-3.2(m, 3H), 3.42-3.52(m, 1...
Embodiment 3
[0059] 1. Synthesis of 2-(4-fluorobutylamino)tetrabenazine (A-3)
[0060] (1) The structure of the compound
[0061]
[0062] (2) Synthesis method
[0063] Dissolve 1.5mmol of 2-aminotetrabenazine in 6ml of DMF, add 1.3mol of 4-fluoro-n-butyl bromide and 1.5mmol of anhydrous potassium carbonate, heat and stir at 80°C for 8 hours, and stop the reaction. Add 150ml of ethyl acetate to dilute the reaction solution and wash with H 2 After washing with O (4×15ml) and saturated sodium chloride solution, it was dried by adding anhydrous sodium sulfate. The solvent was evaporated by filtration and separated by column chromatography. The solvent ratio was: ethyl acetate:n-hexane:dichloromethane:triethylamine=1:9:1:0.5. A yellow oily solid was obtained in 23.4% yield.
[0064] (3) Confirmation of A-3
[0065] h 1 -NMR (CDCl 3 , 200MHz): δ (ppm) 0.88-0.98 (m, 6H), 1.0-1.34 (m, 3H), 1.38-1.80 (m, 7H), 1.98-2.14 (m, 1H), 2.30-2.7 (m, 6H), 2.80-3.2(m, 3H), 3.4-3.52(d, 1H), 3.82-3....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com