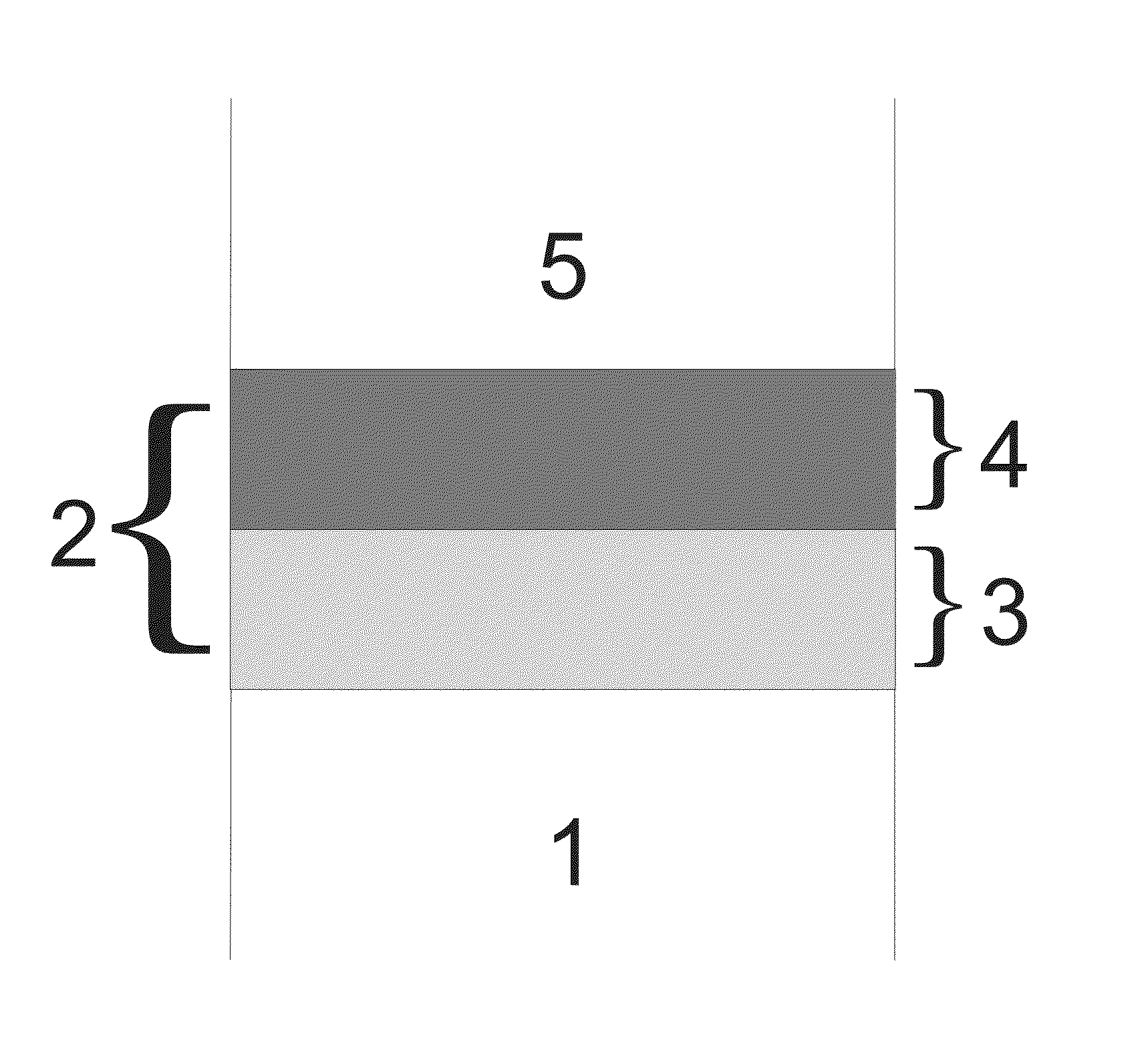
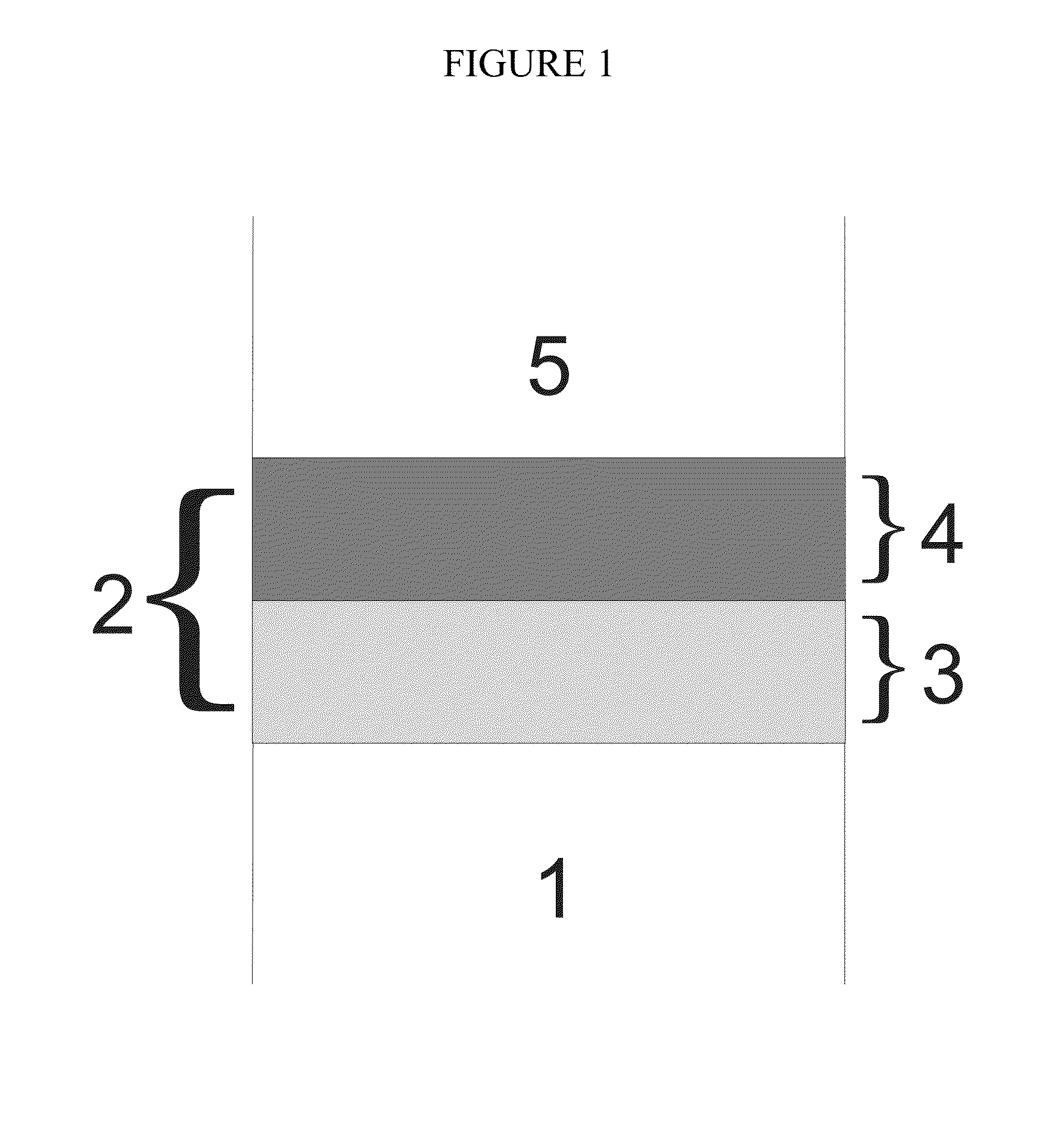
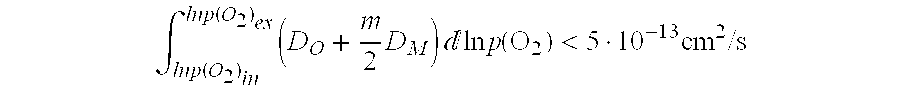Multi-layer coating
a multi-layer coating and coating technology, applied in the superimposed coating process, cell components, electrochemical generators, etc., can solve the problems of increased power loss, high electrical resistance across the interconnect plate, and oxidation on both sides of the metallic interconnect,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0089]A dual layer coating was deposited on a Cr2O3-forming Fe-22Cr alloy. The first oxide layer was deposited by PLD on the alloy surface with the composition La0.95Sr0.05CrO3 with a thickness of 5 μm, said composition having a perovskite structure. Afterwards, a 5 μm MnCr2O4 layer having a spinel structure was deposited on the perovskite layer by PLD so as to form a dual layer coating. The Cr tracer diffusion coefficient for La0.95Sr0.05CrO3 has been measured to be 1.07×10−17 cm2 / s at 1000° C. (N. Sakai et al., Solid State Ionics, 135 (2000) p. 469). The oxygen tracer diffusion coefficient of the MnCr2O4 layer has been measured to be 6×10−15 cm2 / s at 800° C. (N. Sakai et al., Solid State Ionics, 176 (2005) p. 681).
example 2
[0090]A dual-layer coating was formed on a ferritic Fe—Cr interconnect. The first oxide layer was directly deposited on the metal by slurry spraying LaCrO3 having a perovskite structure. Afterwards, a 5 μm thin MnCr2O4 layer having a spinel structure was deposited on the perovskite layer by PLD so as to form the dual-layer coating.
example 3
[0091]A coating as described in Example 2 was formed, followed by deposition of a layer of MnCo2O4 by PLD on top of the spinel layer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| high temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com