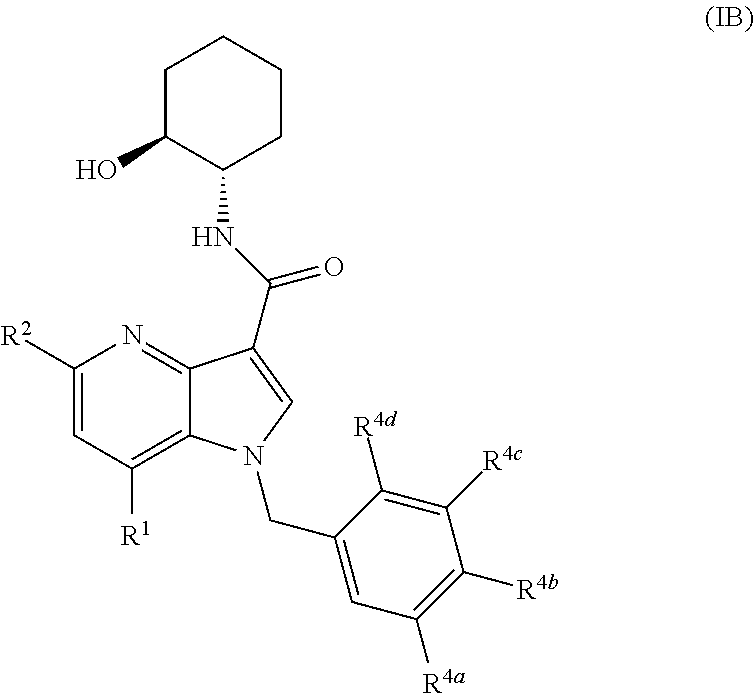
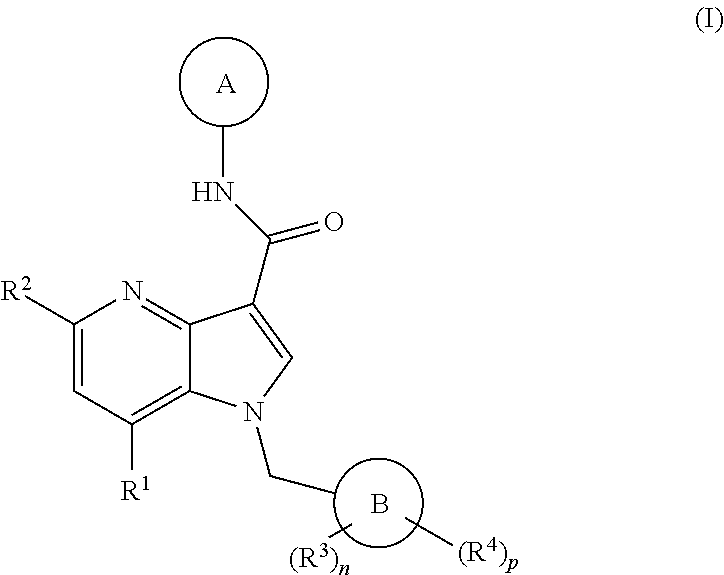
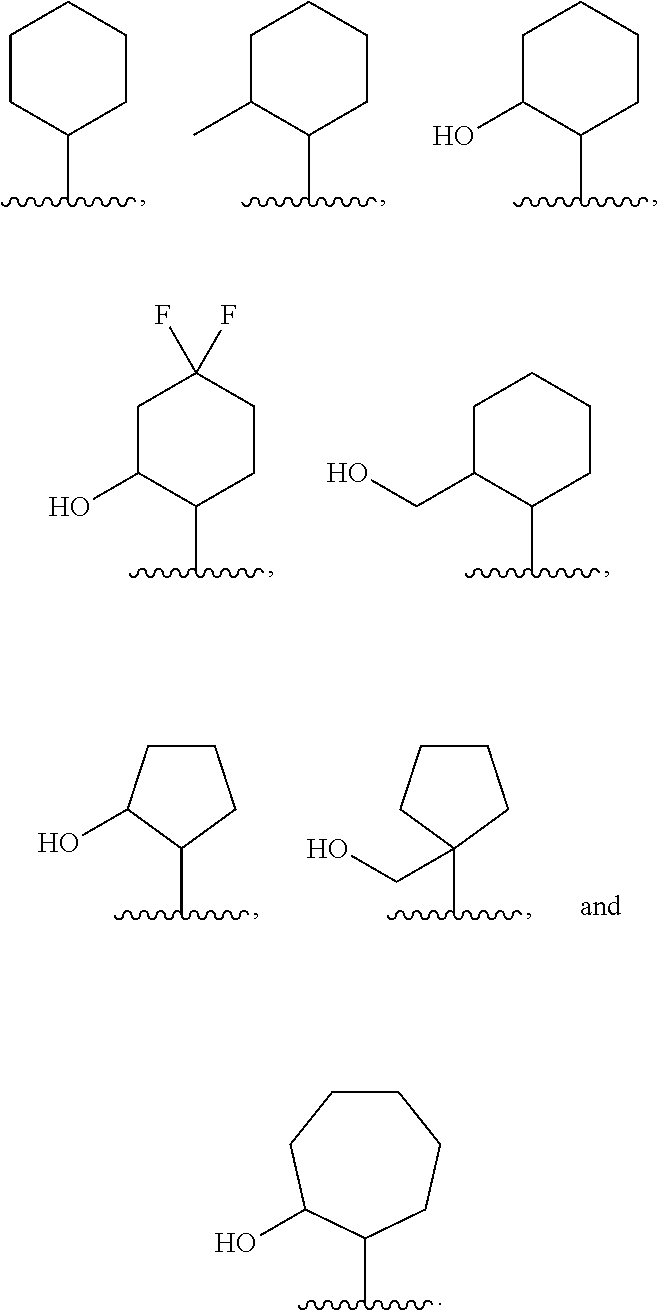
4-Azaindole Derivatives
a technology of azaindole and derivatives, applied in the field of 4azaindole derivatives, can solve the problems of difficult to obtain selective machr m1 orthosteric ligands, side effects of dose-limiting peripheral cholinergics,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples 1-105
Compound Examples 1-105
Example 1
N-((1S,2S)-2-hydroxycyclohexyl)-1-(4-methylbenzyl)-1H-pyrrolo[3,2-b]pyridine-3-carboxamide
[0577]To a mixture of N-((1S,2S)-2-hydroxycyclohexyl)-1H-pyrrolo[3,2-b]pyridine-3-carboxamide (Intermediate 4), (100 mg), 1-(chloromethyl)-4-methylbenzene (60 mg) and cesium carbonate (289 mg) was added DMF (4 mL) and left to stir at rt for 90 min. The crude product was purified by prep. LC-MS to give the desired compound (88 mg).
[0578]LCMS: m / z 364.65 [M+H]+.
[0579]1H NMR (400 MHz, CDCl3) ppm 1.20-1.88 (m, 6H) 2.10-2.21 (m, 2H) 2.34 (s, 3H) 3.57 (td, J=9.9, 4.5 Hz, 1H) 3.80-4.02 (m, 1H) 4.54 (br. s., 1H) 5.30 (s, 2H) 6.99-7.10 (m, 2H) 7.10-7.22 (m, 3H) 7.50-7.72 (m, 1H) 8.09 (s, 1H) 8.39-8.59 (m, 1H) 9.05 (d, J=6.1 Hz, 1H)
example 2
1-(3,5-Difluorobenzyl)-N-((1S,2S)-2-hydroxycyclohexyl)-1H-pyrrolo[3,2-b]pyridine-3-carboxamide
[0580]To a mixture of N-((1S,2S)-2-hydroxycyclohexyl)-1H-pyrrolo[3,2-b]pyridine-3-carboxamide (Intermediate 4), (80 mg), 1-(bromomethyl)-3,5-difluorobenzene (77 mg) and cesium carbonate (231 mg) was added DMF (3 mL) and left to stir at rt over the weekend. The crude product was diluted with MeOH and purified using an SCX-2 cartridge (washed sequentially with MeOH, H2O, MeOH and product eluted using 2 M methanolic ammonia). The solution was evaporated under vacuum to give a solid which was dissolved in DCM and purified using column chromatography (normal phase, 25 g, Biotage SNAP cartridge KP-Sil, 25 mL / min, gradient 0-100% EtOAc in n-hexane) to give the desired compound (70 mg)
[0581]LCMS: m / z 386.59 [M+H]+.
[0582]1H NMR (400 MHz, CDCl3) ppm 1.15-1.67 (m, 4H) 1.72-1.91 (m, 2H) 2.07-2.23 (m, 2H) 3.59 (td, J=9.9, 4.5 Hz, 1H) 3.92 (dddd, J=11.5, 9.4, 7.0, 4.4 Hz, 1H) 5.34 (s, 2H) 6.57-6.69 (m, 2...
example 3
1-(2,5-Difluorobenzyl)-N-((1S,2S)-2-hydroxycyclohexyl)-1H-pyrrolo[3,2-b]pyridine-3-carboxamide
[0583]To a mixture of N-((1S,2S)-2-hydroxycyclohexyl)-1H-pyrrolo[3,2-b]pyridine-3-carboxamide (Intermediate 4), (80 mg), 2-(bromomethyl)-1,4-difluorobenzene (77 mg) and cesium carbonate (231 mg) was added DMF (3 mL) and left to stir at rt over the weekend. The crude product was purified by prep. LC-MS to give the desired compound (68 mg).
[0584]LCMS: m / z 386.59 [M+H]+.
[0585]1H NMR (400 MHz, CDCl3) ppm 1.10-1.64 (m, 4H) 1.67-1.90 (m, 2H) 2.14 (d, J=11.6 Hz, 2H) 3.57 (td, J=9.8, 4.5 Hz, 1H) 3.81-4.00 (m, 1H) 5.36 (s, 2H) 6.57-6.77 (m, 1H) 6.88-7.05 (m, 1H) 7.05-7.14 (m, 1H) 7.22 (dd, J=8.3, 4.7 Hz, 1H) 7.69 (d, J=8.3 Hz, 1H) 8.11 (s, 1H) 8.53 (d, J=4.4 Hz, 1H) 9.04 (d, J=6.2 Hz, 1H)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com