Conjugates of Proteins and Multivalent Cell-Penetrating Peptides and Their Uses
a technology of conjugates and cell penetrating peptides, applied in the field of conjugates of proteins and multivalent cell penetrating peptides and their uses, can solve the problems of affecting the clinical application of novel biological therapeutics based on molecular medicine knowledge, affecting the clinical application and affecting the clinical application success of many high molecular weight drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
1. Materials and Methods
1.1 General.
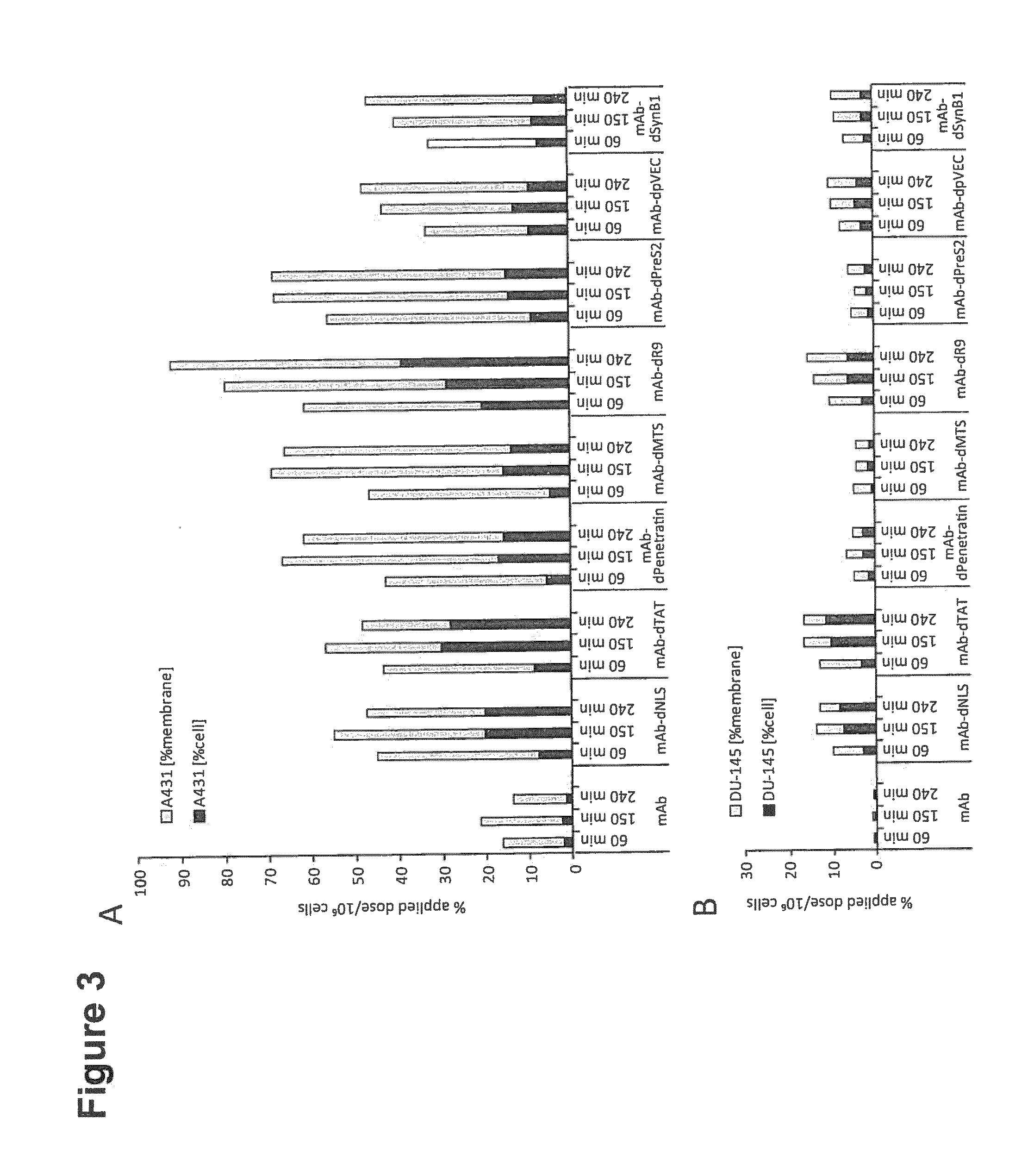
[0209]All chemicals were purchased from Sigma-Aldrich (Schnelldorf, Germany) at the highest available purity unless otherwise stated. Fmoc-protect amino acid building blocks were purchased from Bachem (Bubendorf, Switzerland). Anti-EGFR antibody Matuzumab (EMD72000) was provided by Merck KGaA (Darmstadt, Germany) (see also EP 0 531 472 B1, U.S. Pat. No. 5,558,864, WO 2009 / 043490 A1). Radioactive iodine 1-125 and 1-131 isotopes were purchased from Perkin-Elmer (Rodgau, Germany) and Eckert & Ziegler (Berlin, Germany) for I-124.
1.2 Synthesis of Dendritic Cell-Penetrating Peptides (dCPPs).
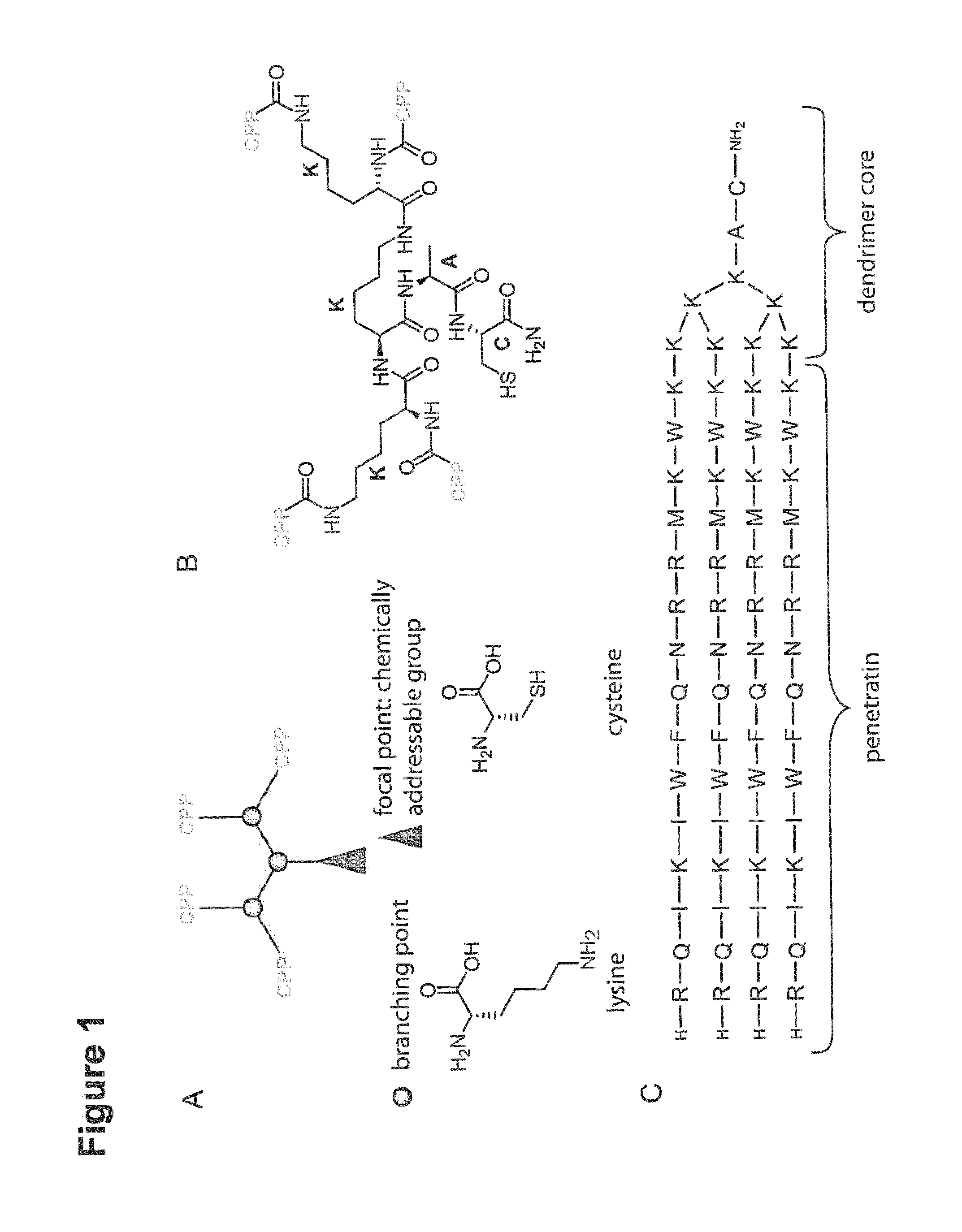
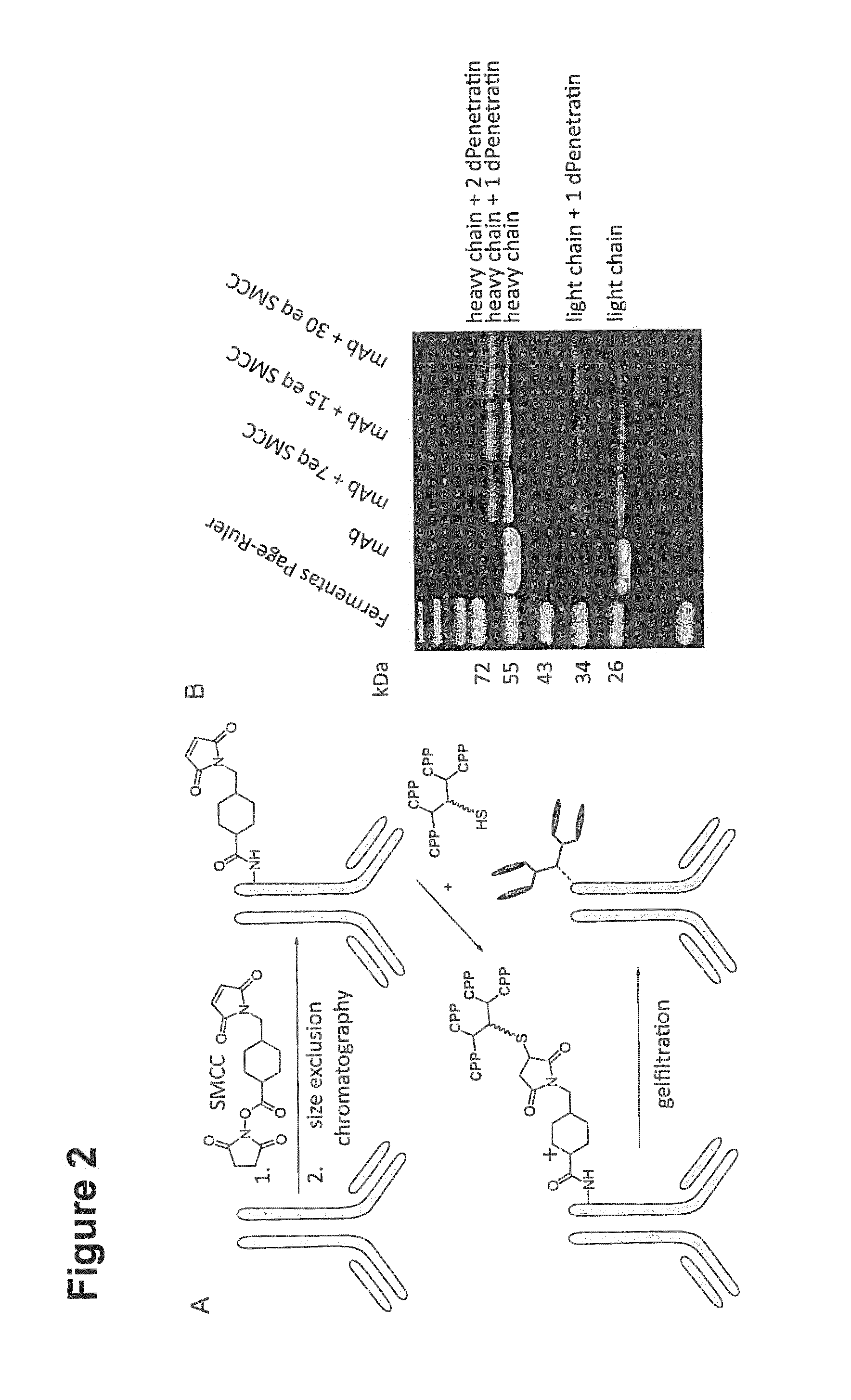
[0210]Branched structures of the CPPs were obtained by manual generation of a solid phase peptide synthesis (SPPS) resin, presenting four amino groups—α- and ε-amines of lysines—as branching points, one alanine residue as spacer and a cysteine as focal group for crosslinking with the antibody using 9-fluorenylmethoxycarbonyl (Fmoc)-protected L-α-amino acids. The desi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com