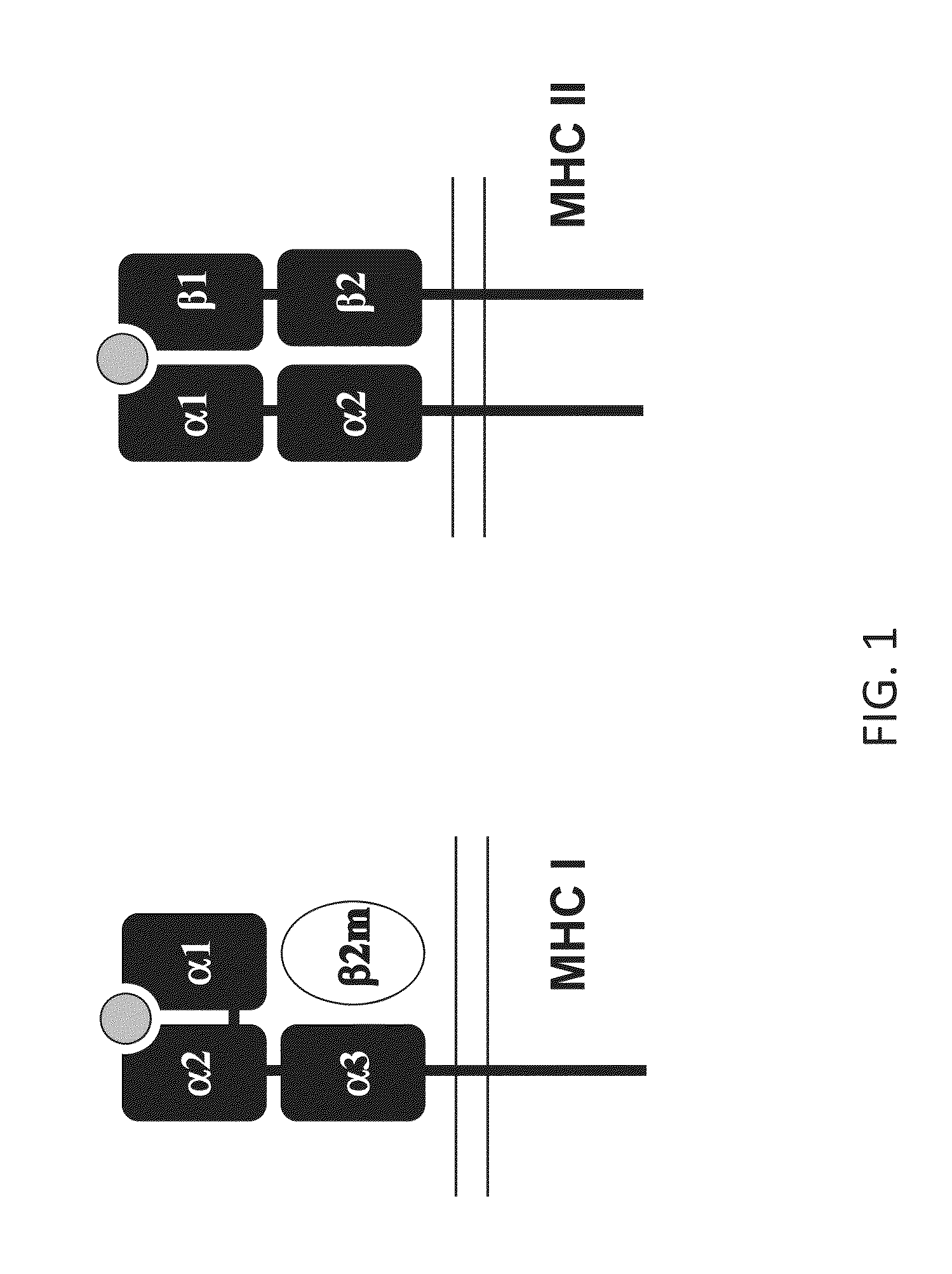
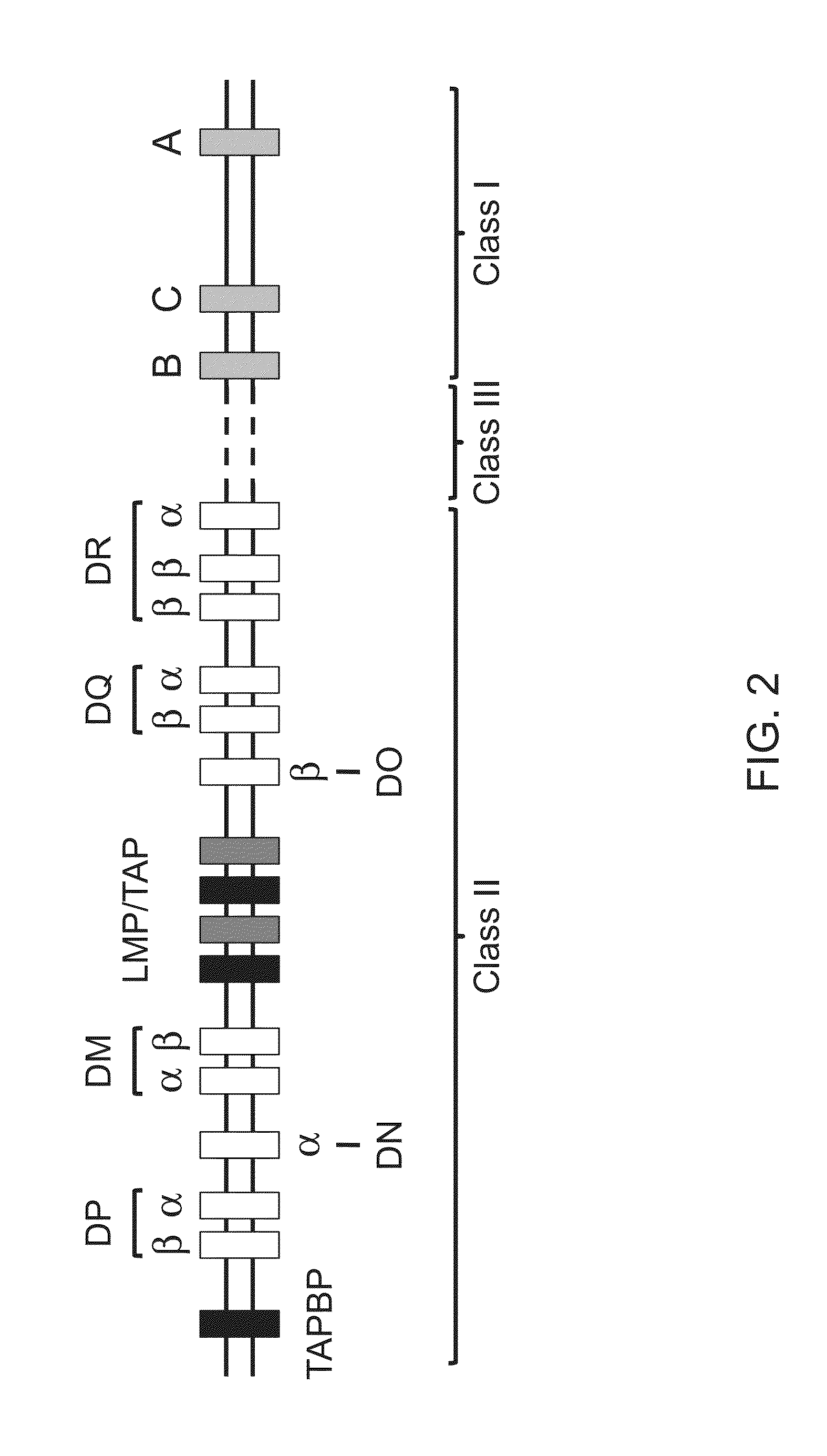
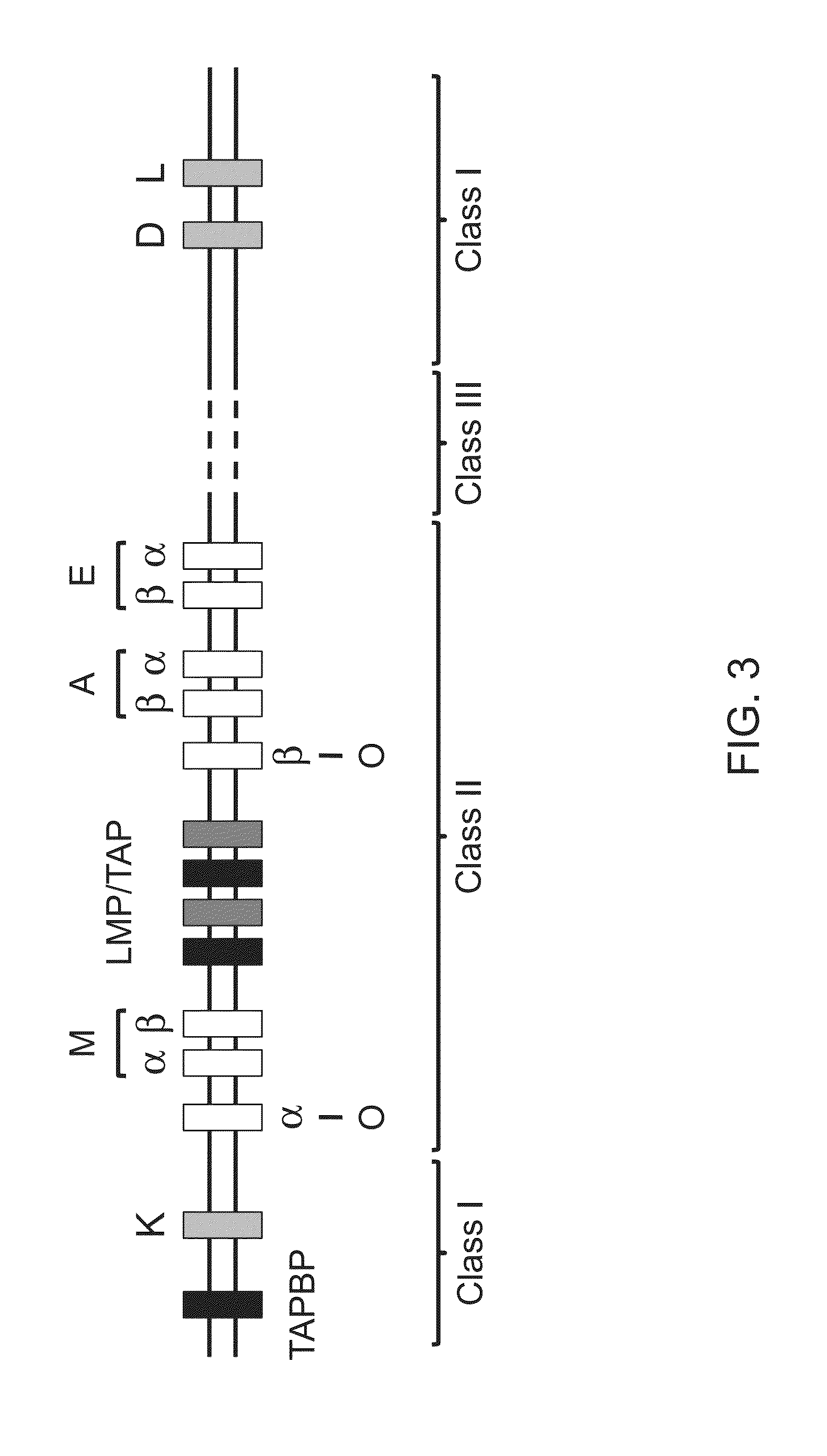
Genetically modified major histocompatibility complex mice
a histocompatibility complex and mouse technology, applied in the field of genetically modified nonhuman animals, can solve problems such as the destruction of cells presenting such peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Engineering a Mouse Comprising Humanized MHC I and MHC II Loci
[0100]The various steps involved in engineering a mouse comprising humanized MHC I and MHC II loci, with corresponding and additional endogenous MHC I and MHC II loci deletions (HLA-A2 / H-2K, HLA-DR2 / H-2E, H-2A-del, H-2D-del) are depicted in FIG. 4. Detailed description of the steps appears below.
example 1.1
Engineering Mouse ES Cells Comprising Humanized MHC I Gene
[0101]The mouse H-2K gene was humanized in a single step by construction of a unique targeting vector from human and mouse bacterial artificial chromosome (BAC) DNA using VELOCIGENE® technology (see, e.g., U.S. Pat. No. 6,586,251 and Valenzuela et al. (2003) High-throughput engineering of the mouse genome coupled with high-resolution expression analysis. Nat. Biotech. 21(6): 652-659). DNA from mouse BAC clone RP23-173k21 (Invitrogen) was modified by homologous recombination to replace the genomic DNA encoding the α1, α2 and α3 domains of the mouse H-2K gene with human genomic DNA encoding the α1, α2 and α3 subunits of the human HLA-A2 gene (FIG. 5). Detailed steps for construction of BAC DNA can be found in U.S. Patent Application Publication No. 2013-0111617, incorporated herein by reference. The targeted BAC DNA was used to electroporate mouse F1H4 ES cells to create modified ES cells for generating mice that express a chim...
example 1.2
Engineering Mouse ES Cells Comprising Deletion of MHC II Loci
[0103]The targeting vector for introducing a deletion of the endogenous MHC class II H-2Ab1, H-2Aa, H-2Eb1, H-2Eb2, and H-2Ea genes was made using VELOCIGENE® genetic engineering technology (see, e.g., U.S. Pat. No. 6,586,251 and Valenzuela et al., supra). Bacterial Artificial Chromosome (BAC) RP23-458i22 (Invitrogen) DNA was modified to delete the endogenous MHC class II genes H-2Ab1, H-2Aa, H-2Eb1, H-2Eb2, and H-2Ea.
[0104]Briefly, upstream and downstream homology arms were derived by PCR of mouse BAC DNA from locations 5′ of the H-2Ab1 gene and 3′ of the H-2Ea gene, respectively. These homology arms were used to make a cassette that deleted ˜79 kb of RP23-458i22 comprising genes H-2Ab1, H-2Aa, H-2Eb1, H-2Eb2, and H-2Ea of the MHC class II locus by bacterial homologous recombination (BHR). This region was replaced with a neomycin cassette flanked by lox2372 sites. The final targeting vector included from 5′ to 3′ a 26 kb ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| polymorphic | aaaaa | aaaaa |
| non-covalent | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com