VAL66MET (SNP rs6265) GENOTYPE SPECIFIC DOSING REGIMENS AND METHODS FOR THE TREATMENT OF DEPRESSION
a genotype-specific, depression-specific technology, applied in the direction of biochemical apparatus and processes, drug compositions, biocide, etc., can solve the problems of clinically significant distress or impairment in social, occupational or other important areas of functioning, psychological distress, and the onset of suicidal ideation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Effect of Val66Met rs6265 Polymorphism in BDNF; Retrospective Analysis of Esketamine (ESKETIVTRD2001) and Ketamine (KETIVTRD2002) Clinical Trials
ESKETIVTRD2001 Clinical Trial Design and Objectives:
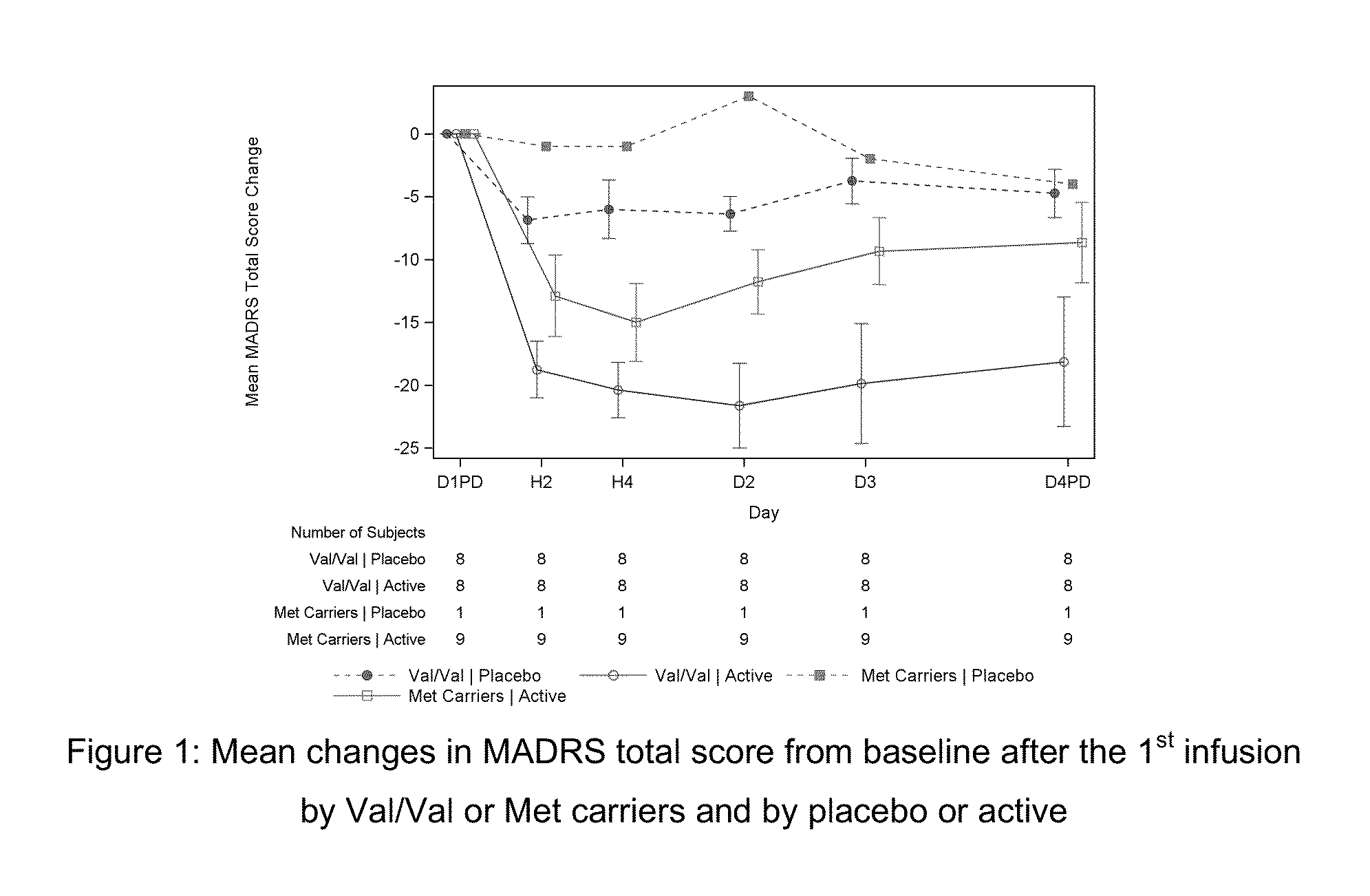
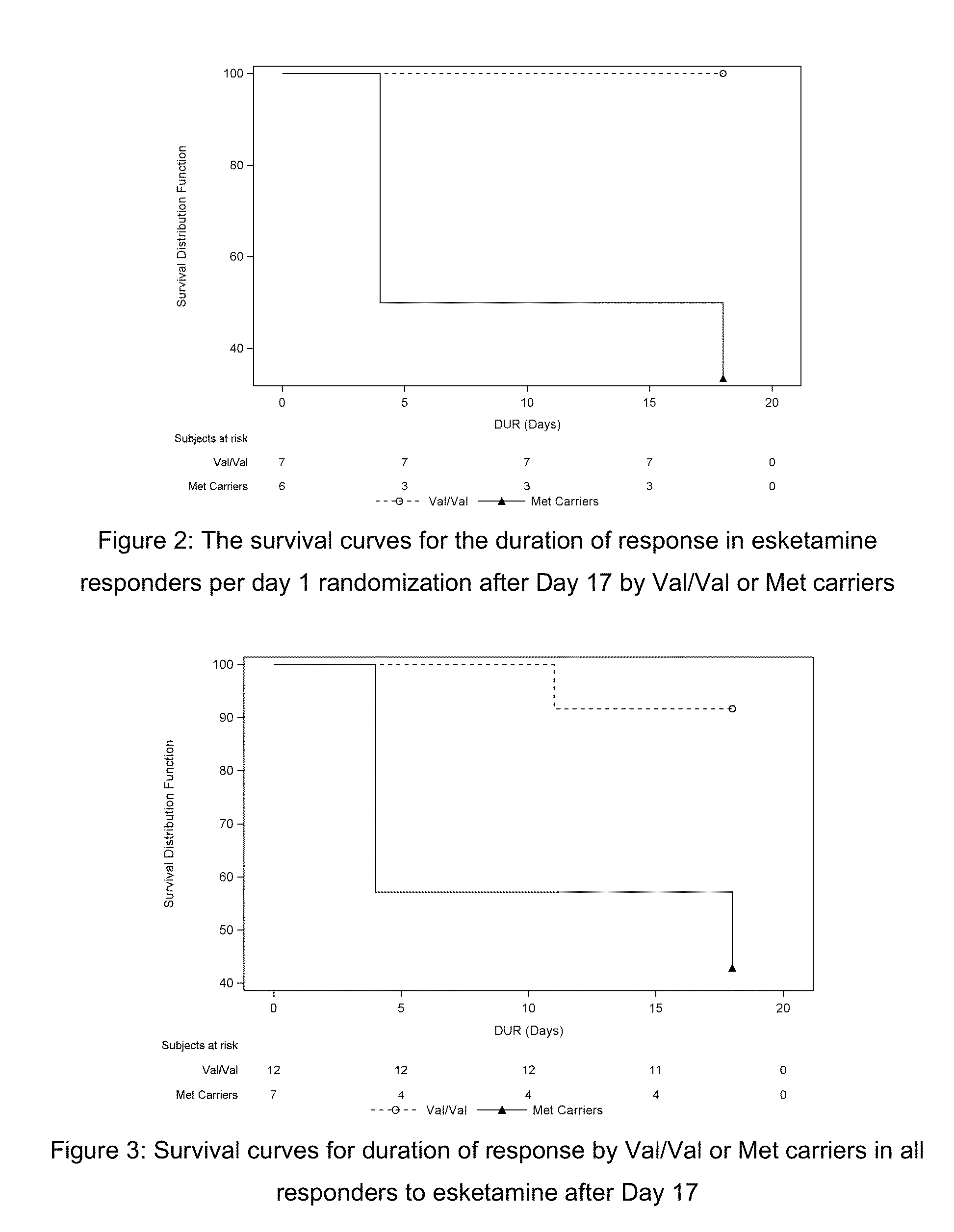
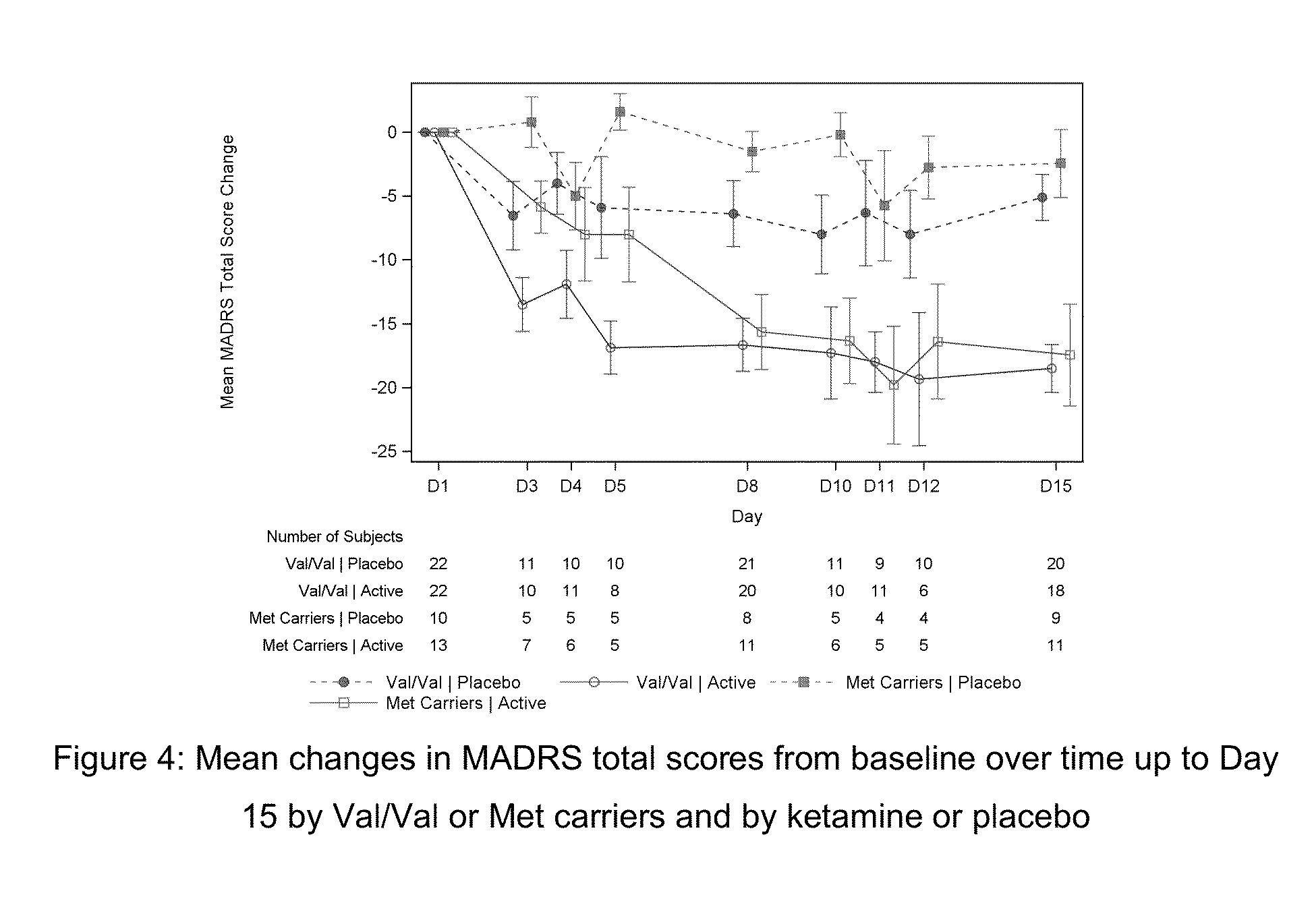
[0144]This was a double-blind, double-randomization, placebo-controlled, multiple dose titration study in 30 adult subjects with TRD. The study consisted of 3 phases: a screening phase of up to 2 weeks, a 7-day double-blind (DB) treatment phase (Day 1 to Day 7), and a 4-week post-treatment (with optional open label [OL] esketamine 0.40 mg / kg during follow-up [FU]: administered on Days 7, 10, 14 and 17.). The interval between the first and last dose of study medication was 3 days. Approximately 30 adult subjects with TRD were randomized to treatment (esketamine 0.40 mg / kg, esketamine 0.20 mg / kg, or placebo i.v. infusion) in a 1:1:1 ratio on Day 1.
[0145]If esketamine 0.40 mg / kg dose was not well tolerated on Day 1 and / or Day 4 the dose may be reduced to 0.3 mg / kg. Subjects who were responder...
formulation example 1
Prophetic Example
[0200]An aqueous formulation of S-ketamine hydrochloride is prepared by mixing S-ketamine hydrochloride (at a concentration of 161.4 mg / mL) in water and then adding 1N NaOH(aq) to pH 5.0.
formulation example 2
Prophetic Example
[0201]Aqueous formulation of S-ketamine hydrochloride is prepared by mixing S-ketamine hydrochloride (at a concentration of 161.4 mg / mL) in water and then adding 10 mg / mL tauroursodeoxycholic acid (TUDCA). To the resulting mixture is added 1N NaOH(aq) to pH 4.5.
[0202]While the foregoing specification teaches the principles of the present invention, with examples provided for the purpose of illustration, it will be understood that the practice of the invention encompasses all of the usual variations, adaptations and / or modifications as come within the scope of the following claims and their equivalents.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| dosing frequency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com