Methods and formulations for treating vascular eye diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
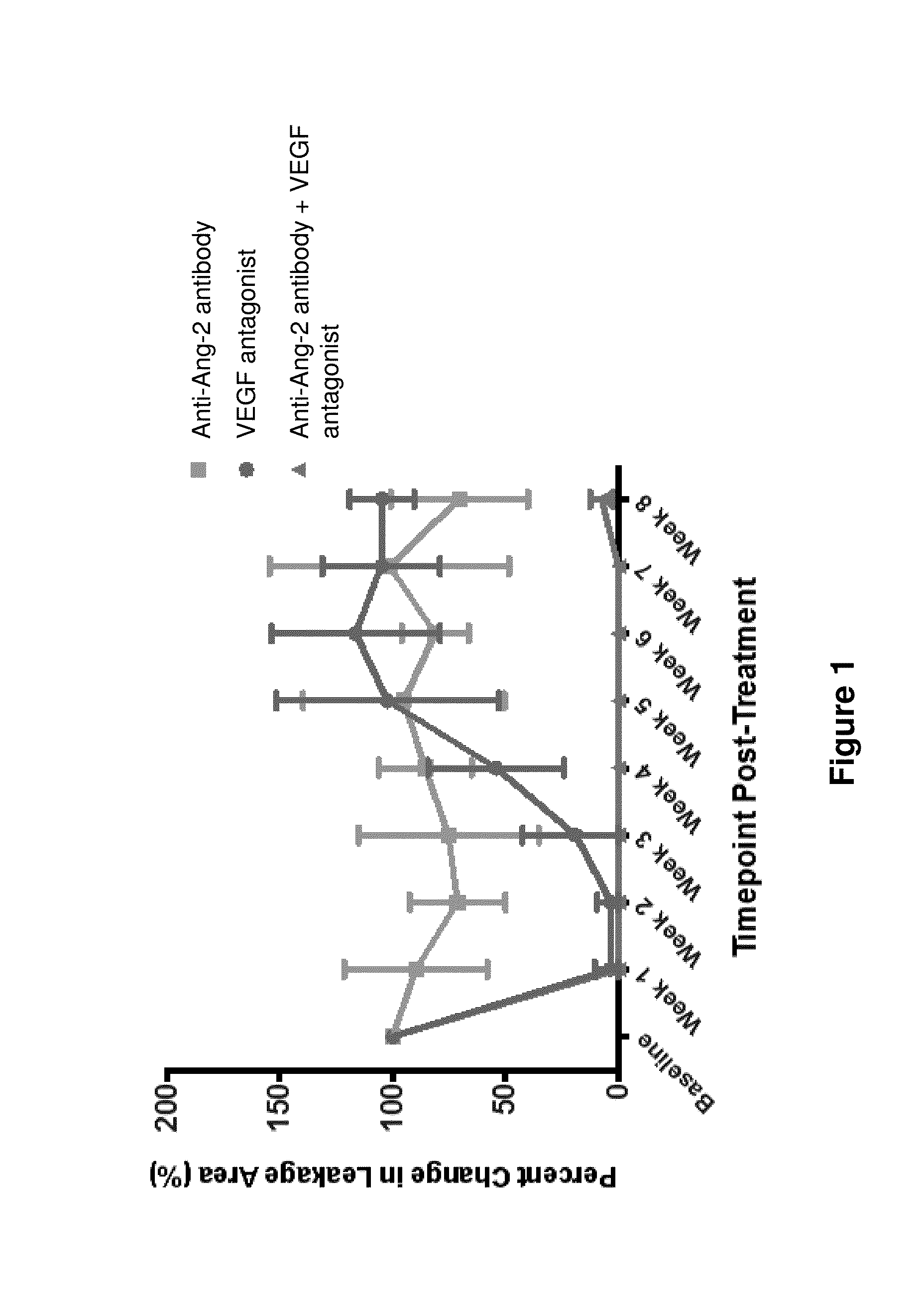
Effects of Combined Inhibition of VEGF and Ang2 Using Aflibercept (VEGF Trap) and Anti-Ang2 Antibody on the Developing Retinal Angiogenesis in Mice
[0141]Introduction: VEGF is the key modulator of angiogenesis in normal and pathological angiogenesis. However, other growth factors are also involved in angiogenesis and are able to mediate blood vessel resistance to anti-VEGF therapies. Angiopoietin-2 (Ang2) was shown to be involved in blood vessel growth and regression in various circumstances in a context-dependent manner. In this study we tested the effects of VEGF blockade using aflibercept alone and in combination with Ang2 inhibition with an anti-Ang2 antibody on blood vessel growth and regression in a normal retinal vascular development (RVD) model.
[0142]Human anti-Ang-2 antibodies were generated as described in US Patent Application Publication No. US20110027286. The exemplary anti-Ang-2 antibody used in the present and following Examples is the human anti-Ang-2 antibody designa...
example 2
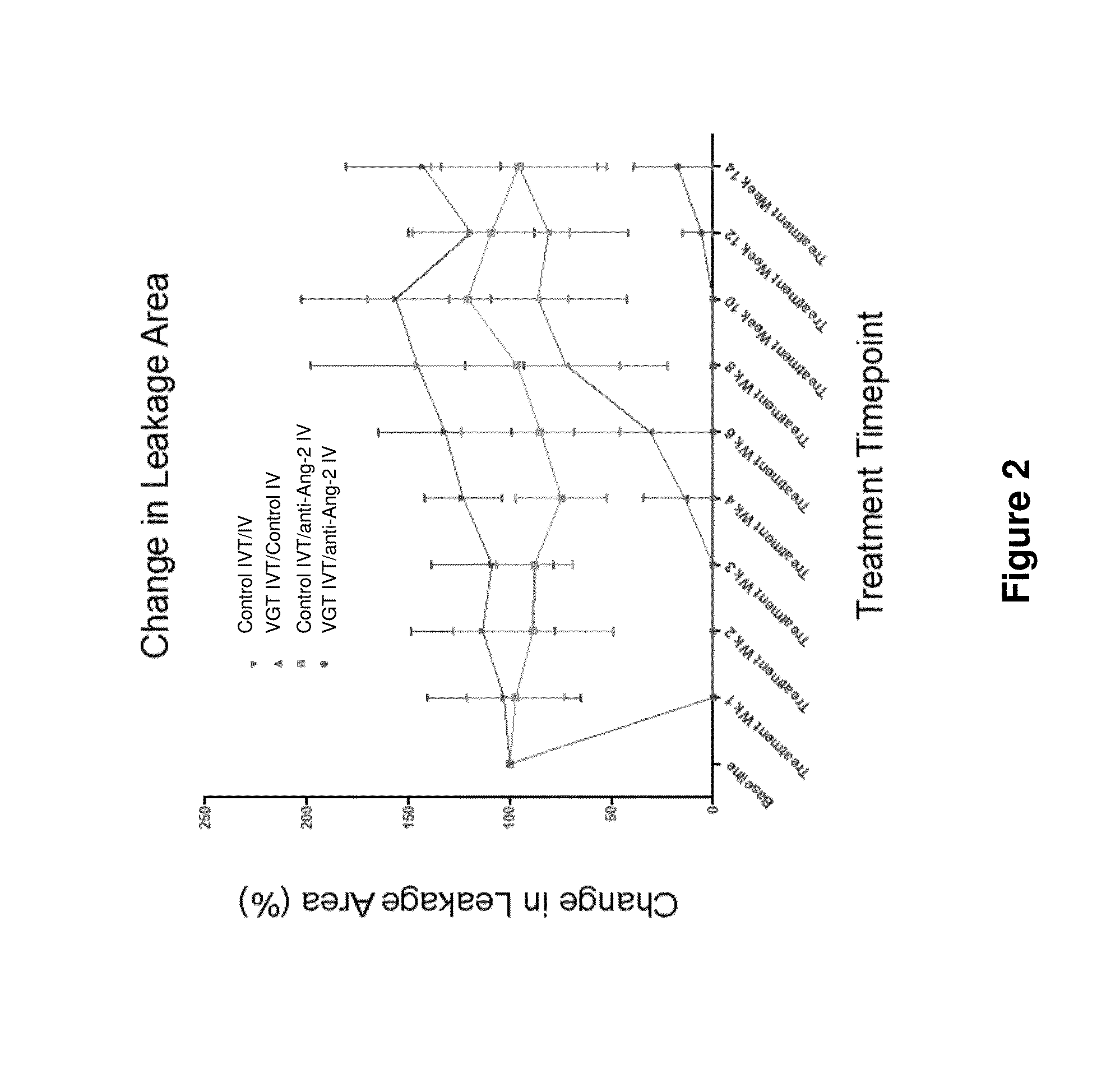
Effect of IVT Injection of Co-Formulated mAb1 and Aflibercept on DL-Alpha-Aminoadipic Acid (DL-Alpha-AAA)-Induced Retinal Neovascularization (RNV) in Rabbit Eyes
[0148]The glial toxin DL-alpha-AAA targets retinal Muller cells and astrocytes and leads to neovascularization and chronic vascular leak lasting at least 12 months (Kato et al 1993; Neuroscience 57: 473). The purpose of this study was to evaluate the effects of IVT injection of co-formulated Anti-Ang2 and aflibercept on RNV induced by DL-alpha-AAA.
[0149]Methods: Male New Zealand White Rabbits (>2 kg body weight) were treated with a single intravitreal injection of DL-alpha-AAA to induce RNV. The leak was monitored and quantitated non-invasively using fluorescein angiography (FA). Pathological vasculature and a relatively invariable leakage area were established over 13 weeks post injection. Upon disease establishment, the subjects were split into three groups for treatment, as shown below:
[0150]Group I: 125 μg / 50 μl of aflib...
example 3
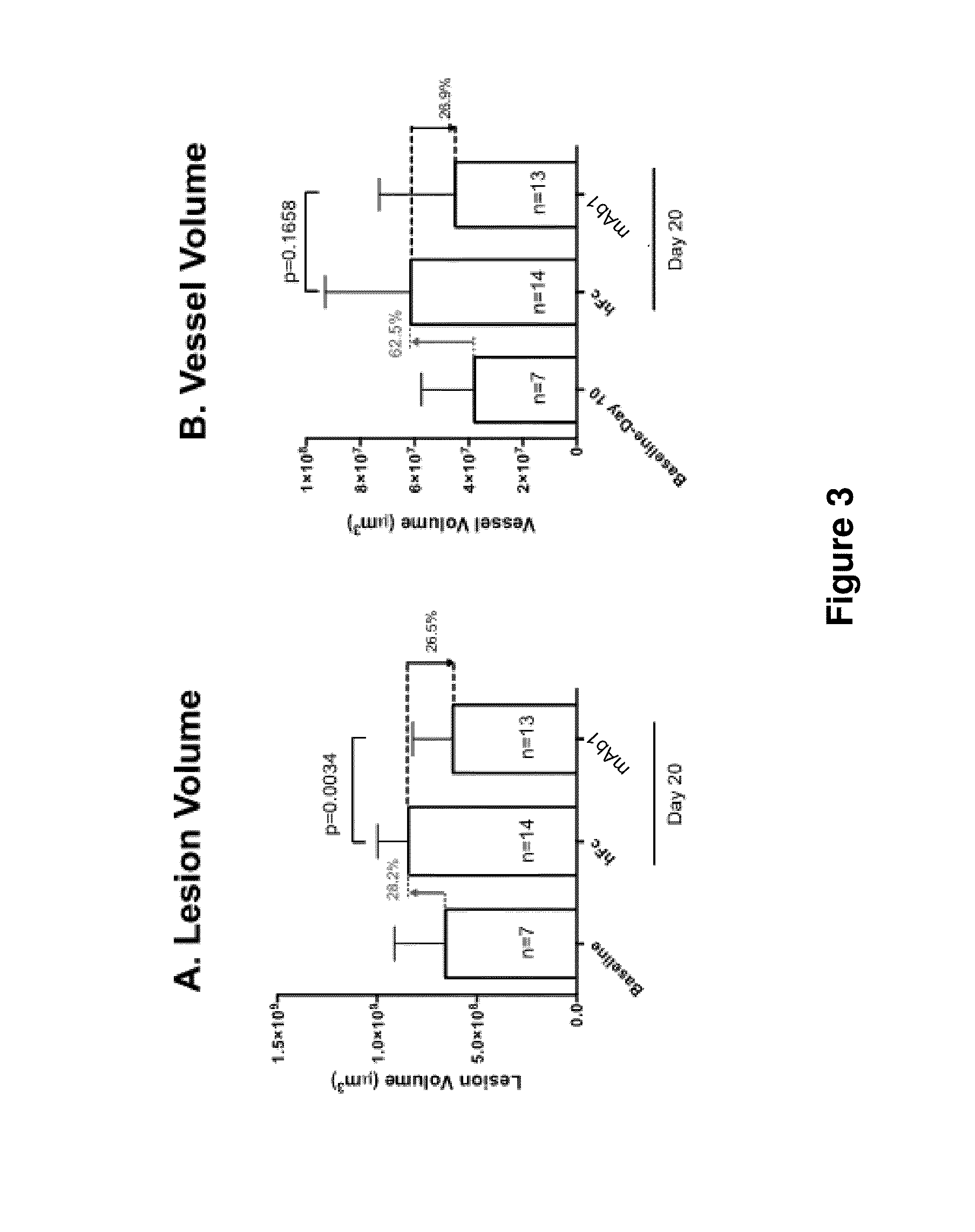
Systemic Administration of mAb1 Alone or Co-Treatment with VEGF Trap-Eye IVT on DL-AAA Induced Retinal Neo-Vascular Leak in Rabbit Eyes
[0155]The purpose of this study was to evaluate the effects of co-treatment of Anti-Ang2 antibody and aflibercept on RNV induced by DL-alpha-AAA, wherein the intravitreal administration of aflibercept was followed by systemic administrations of mAb1. The rationale of this study was to try and maintain the suppression of leakage over longer periods of time with an initial IVT injection of aflibercept and follow up with systemic injections once in two weeks (q2w) of anti-Ang 2 antibody.
[0156]As disclosed in Example 2, retinal neovascularization was induced in male New Zealand rabbits with a single intravitreal injection of DL-alpha-AAA. Stable retinal neovascularization and vascular leak was established 10 weeks post induction. After ten weeks of disease establishment, the subjects were split into four groups with balanced leakage severity and treatmen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com