Anti-pd-l1 combinations for treating tumors
a combination therapy and tumor technology, applied in the direction of antibody medical ingredients, drug compositions, peptides, etc., can solve the problems of signal abrogation, cell death, and difficult to achieve long-term efficient inhibition and killing of tumor cells by the simple majority of antibodies, and the current antibody-drug conjugate drugs are limited in how to kill tumor cells directly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0409]Tumour Inoculation and Evaluation of Tumour Growth
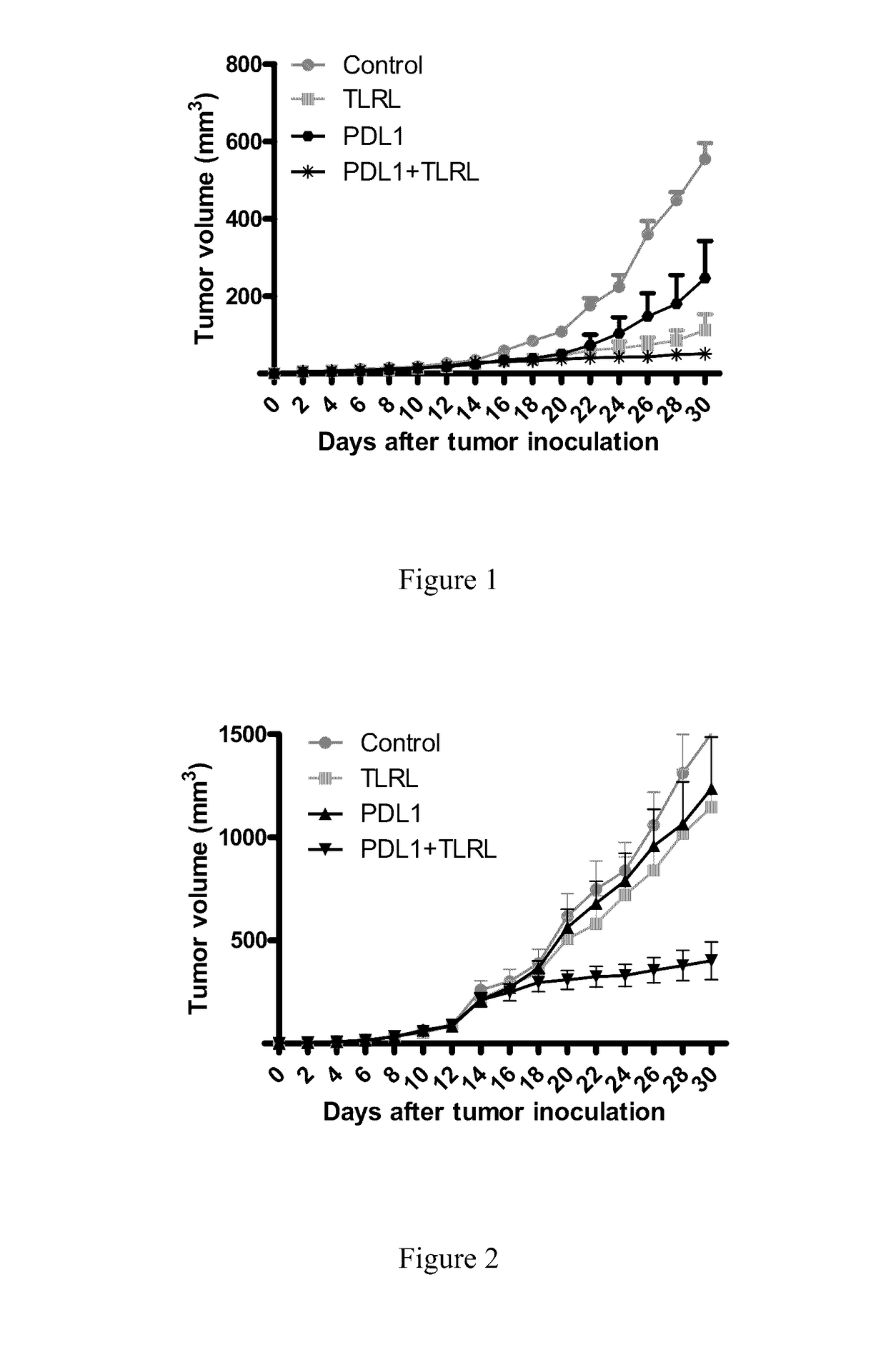
[0410]Mice: Female 6-week-old BALB / c and C3H / HeN (C3H) mice were purchased from Japan SLC (Hamamatsu, Japan). All procedures were reviewed and approved by the Animal Care and Use Committee of the Tokyo Medical and Dental University. The SCCVII (C3H-originated, 3×105), or Colon26 (BALB / c-originated, 5×105) parental cells were injected subcutaneously (s.c.) into the shaved right flank of syngeneic mice and tumour volumes were evaluated. In experiments examining the effects of anti-PDL1(MIH5) mAb with TLRL, 200 ug of anti-PDL1 mAb or 200 ug of anti-PDL1 mAb mixture with TLRL or control rat IgG was injected i.p. three times a week after tumour inoculation. Tumor volumes were measured along three orthogonal axes (x, y, and z) and calculated as tumor volume=(xyz) / 2, if a mouse lose more than 20% of body weight or is very sick and cannot get to adequate food or water, it will be removed from the study and euthanized. (FIGS. 1 and 2)
example 2
[0411]Enrichment of Human Dendritic Cells (DCs) from PBMC
[0412]Human PBMC was prepared from Buffy coats obtained from healthy volunteer donors by Ficoll centrifugation. Dendritic cells were enriched by using negative depletion with magnetic beads (Miltenyi Biotec Inc. San Diego, Calif.) with mixture of anti-CD3, CD19, CD20, CD14, and CD16 antibodies from human PBMC. The enrichment of DCs was stained with goat anti-mouse FITC (lineages), HLA-DR-APCCy7, CD123-BV421 and CD11C-APC. The stained cells were analyzed on BD LSR Fortessa (BD Biosciences). The anti-CD3, CD4, CD11C, CD19, CD14, CD16, CD123 monoclonal antibody were purchased from BD Biosciences, CA or Biolegend, San Diego, Calif.
[0413]Stimulation of Enriched Human DCs and Cytokines Expression
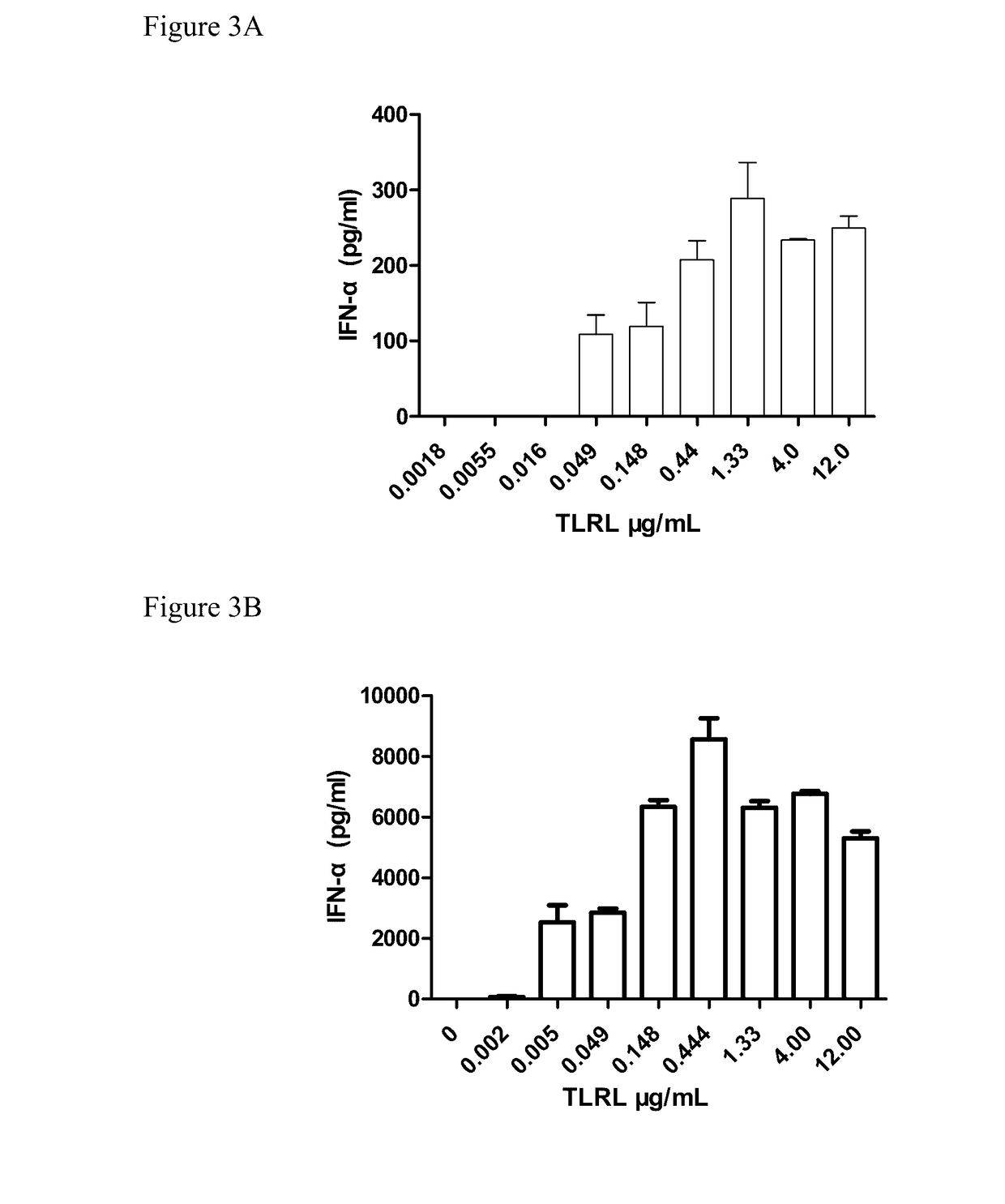
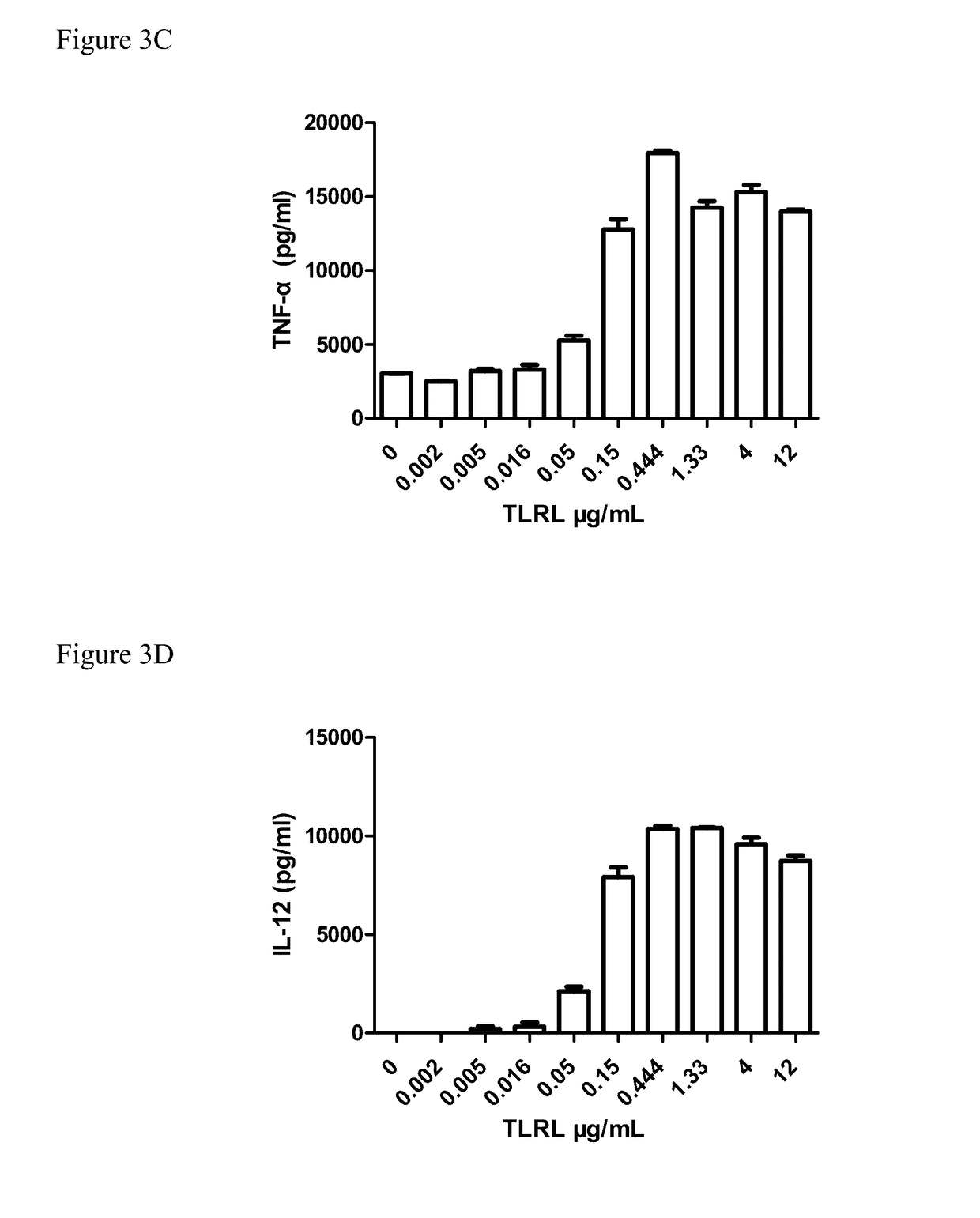
[0414]1-2×105 enriched DCs were plated in a 96-well plate in 100 μl media, 100 μl diluted stimulators (including TLRL were add to the plate and cultured for 20-22h in 37° C. incubator. The supernatant were collected and human IFN-α, IL-12(p7...
example 3
[0416]Detection of Systemic Immune Activation with IFN Inducible Genes Expression in Mouse PBMC by TLRL
[0417]Balb / c mice, 6-8 weeks of age, female, purchased from Vital River were injected intravenously with TLRL, at indicated time point, mice were bled and IFN inducible genes were examined by qPCR. Once pick time of expression IFN inducible genes was determined, a separated experiment was performance with various dose of TLRL. At indicated time point, mice were bled and IFN inducible genes were examined. The Quantitative Real-Time PCR was performed and gene expression data were normalized relative to geometric mean of two housekeeping genes (Actin):
Mouse Actin: (SEQ ID NO.: 1)F: CATTGCTGACAGGATGCAGAAGG,Mouse Actin (SEQ ID NO.: 2)R: TGCTGGAAGGTGGACAGTGAGG;Mouse Inf-b: (SEQ ID NO.: 3)F: CTCCAGCACTGGGTGGAATG,Mouse Inf-b (SEQ ID NO.: 4)R: AGTGGAGAGCAGTTGAGGAC;Mouse Mx2: (SEQ ID NO.: 5)F; GTGGCAGAGGGAGAATGTCG,Mouse Mx2(SEQ ID NO.: 6)R: TAAAACAGCATAACCTTTTGCG;Mouse Ifn-a: (SEQ ID NO.: 7)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameters | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com