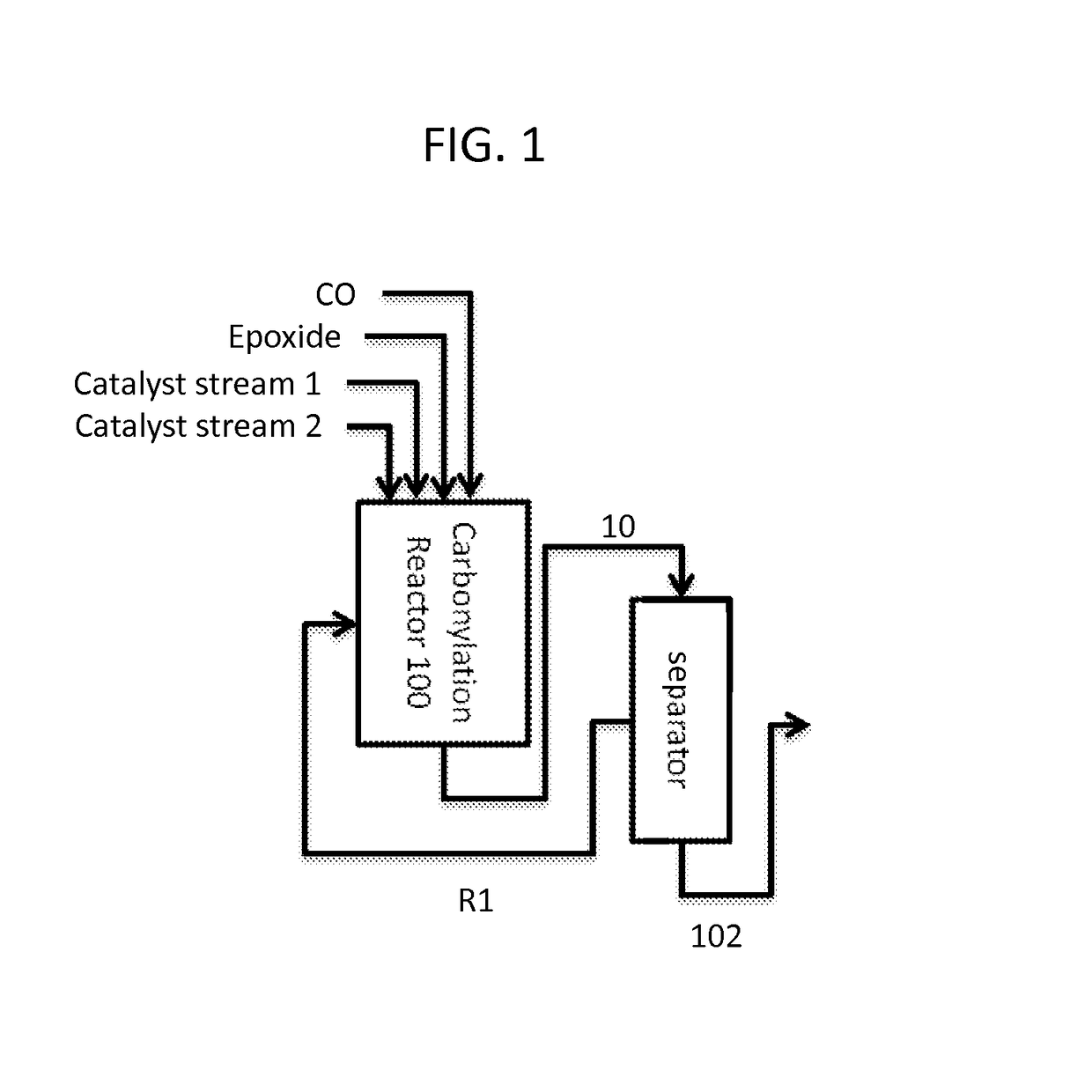
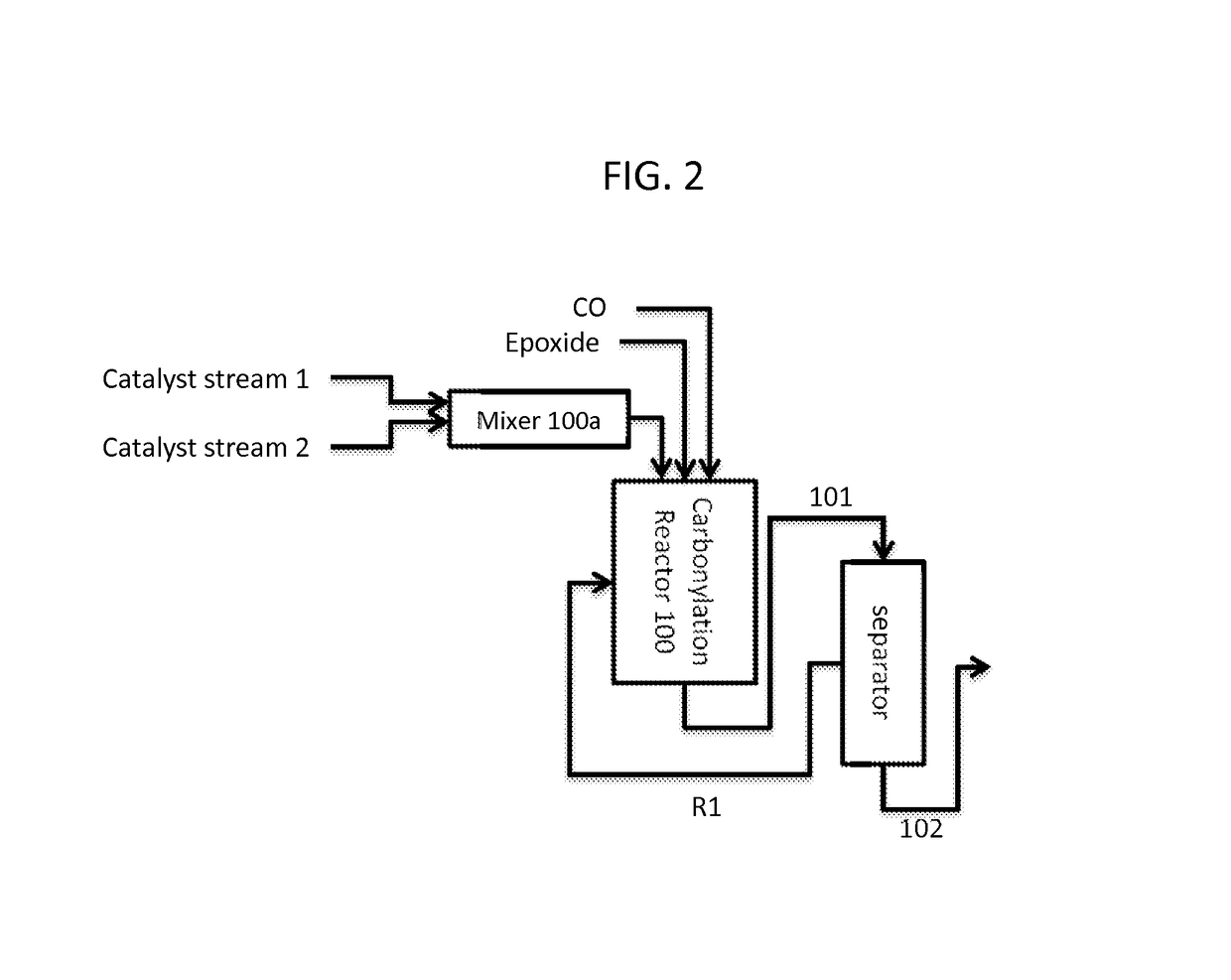
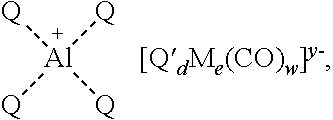Synthesis of metal complexes and uses thereof
a metal complex and metal complex technology, applied in the direction of organic compound/hydride/coordination complex catalyst, organic compound/compound/complex catalyst, group 3/13 element organic compound, etc., can solve the problem of large-scale implementation impracticality, and the alkali metal salt of carbonyl cobaltate utilized for synthesis is not commercially availabl
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 3
ntent of Aluminum Complexes
[0264]Using ICP-AES, the sodium content of the products of Examples 1 and 2 was measured and found to be below the detectable limit. In contrast, the sodium content of the same complexes prepared by salt metathesis were found to have sodium wt % in the range of 0.04 to 0.23 for 10 different preparations.
example 4
of Ketones
[0265]In an NMR spectrum of the reaction product resulting from treatment of (TPP)AlEt with 0.5 eq. of Co2(CO)8, 0.24 molar equiv. of pentanone (relative to the catalyst) was observed in the 1H NMR spectrum after the reaction. In addition, butane was detected in the volatiles from the filtrate and hexanes wash of the crystallized catalyst.
example 5
l Syntheses
[0266]Using the procedures described in Examples 1 and 2, the aluminum complexes [(OEP)Al(THF)2][Co(CO)4] and [(MeOTPP)Al(THF)2][Co(CO)4] can also be made by starting with the appropriate aluminum ligand (i.e., octaethylporphyrin or 5,10,15,20-tetrakis(4-methoxyphenyl)porphyrin).
PUM
| Property | Measurement | Unit |
|---|---|---|
| pressure | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
| pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com