Chimeric activators: quantitatively designed protein therapeutics and uses thereof
a technology of chimeric activators and protein therapeutics, applied in the field of chimeric proteins, fusion proteins, and therapeutic proteins, can solve the problems that erythropoietin can also have serious side effects, and achieve the effect of reducing cell activation properties and avoiding or reducing unwanted side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
GF Chimeric Activator
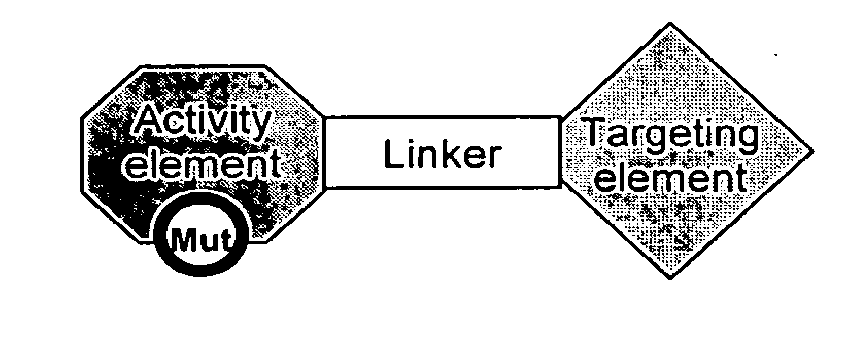
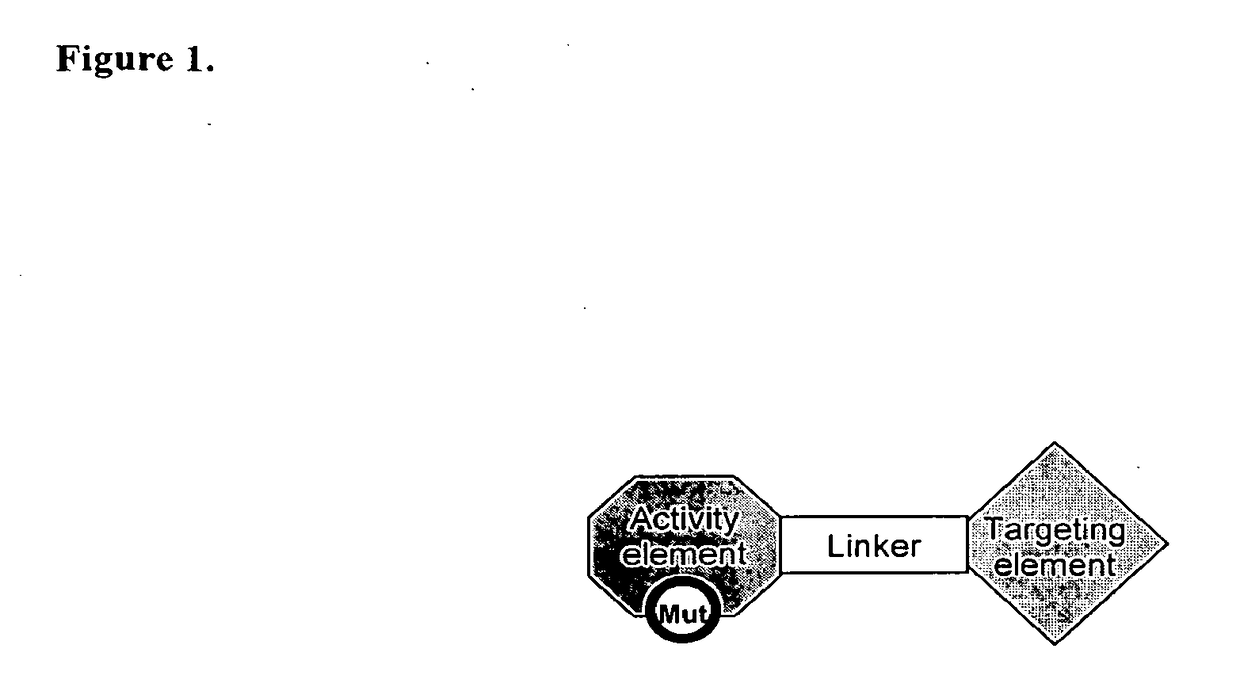
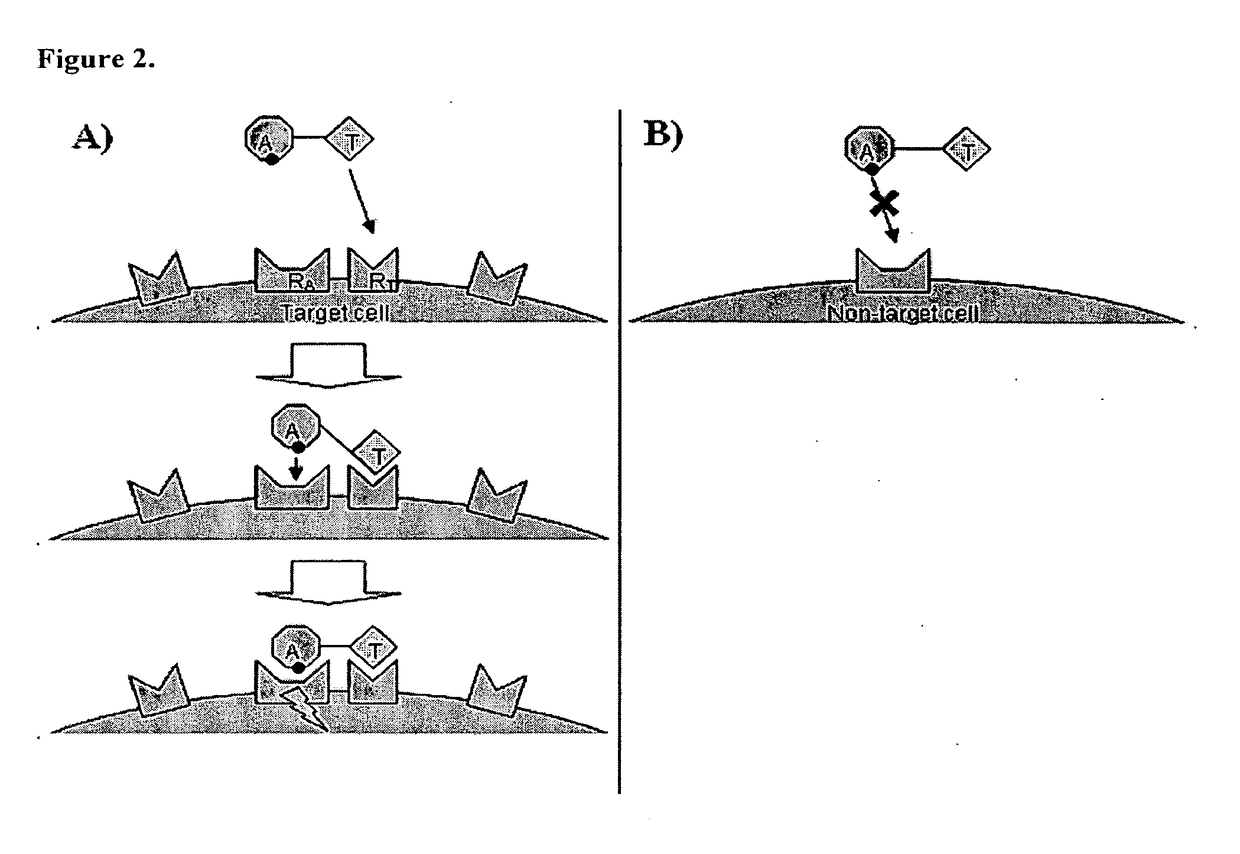
[0121]In some embodiments, a Chimeric Activator protein was constructed with the following protein components: a mutated version of interferon alpha (IFNα) as the Activating Element, a linker, and epidermal growth factor (EGF) as the targeting element. An embodiment of this chimeric protein is illustrated in FIG. 3. In FIG. 3, the IFNα activity element is shown in region (1), the (Gly4Ser)7 linker is shown in region (2), and the EGF targeting element is shown in region (3). In FIG. 3, the Chimeric Activator is bound to both IFNαR2 (shown as region (4)) and an active EGFR dimer (shown as region (5)). The figure shows only the extracellular domains of the receptors. The membrane-spanning domains would be at the bottom of the figure.
[0122]These elements were chosen based on the following considerations. The published structure of EGF receptor with a ligand (Garrett et al. (2002) Cell 110: 763-773) and a model of the IFNα structure (Quadt-Akabayov et al. (2006) Prot...
example 2
Activator in an Animal Tumor Model
[0135]Chimeric Activators of Example 1 can be tested / screened for activity in animal tumor models. Pharmaceutical Product Development, Inc. (New York, N.Y.) can be used as a contract testing service to perform these tests. This type of experiment is standard in the development of anti-cancer drugs, and involves injection of human tumor cells into mice that have immune-suppressing mutations so that the tumor cells will not be rejected. In one form of the experiment, tumor cells are injected subcutaneously and then grow into a lump that can be measured with calipers. Once a tumor has reached a measurable size, treatment is started and the extent of tumor growth inhibition or shrinkage can be charted with time.
[0136]Using IFNα(R149A)-EGF as the test molecule, experiments can be performed in parallel sets of animals used paired tumor cell lines, one of which expresses the receptor for the targeting element, and one of which does not. The Daudi and Daudi...
example 3
rosis Factor α (TNFα) as an Activity Element
[0146]To demonstrate the breadth of the Chimeric Activator concept, each element (activity element, a linker, and a targeting element) of the constructs of Example 1 can be replaced with an alternative component that is structurally different and / or has a different biological function. This is illustrated in this example and those that follow. These experiments may provide a molecule for testing in animals.
[0147]To demonstrate that IFNα can be replaced by other activators of signal transduction, a series of proteins with wild-type and mutant versions of Tumor Necrosis Factor (TNFα) can be constructed. Specifically, the following series of mutations defined by Zhang et al. ((1992) J Biol Chem. 267: 24069-24075) can be used:
MutationRelative activityWild-type100%N39Y30%S147Y7%Y87H0.6%
[0148]TNF is a trimer with a completely different structure from IFNα. The structure of the TNF / TNF receptor complex has been solved. The N-terminus and C-termin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com