Compositions for use in the treatment of diabetes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Expression of GPR84 in BRIN-BD11 Cells and Mouse Islets
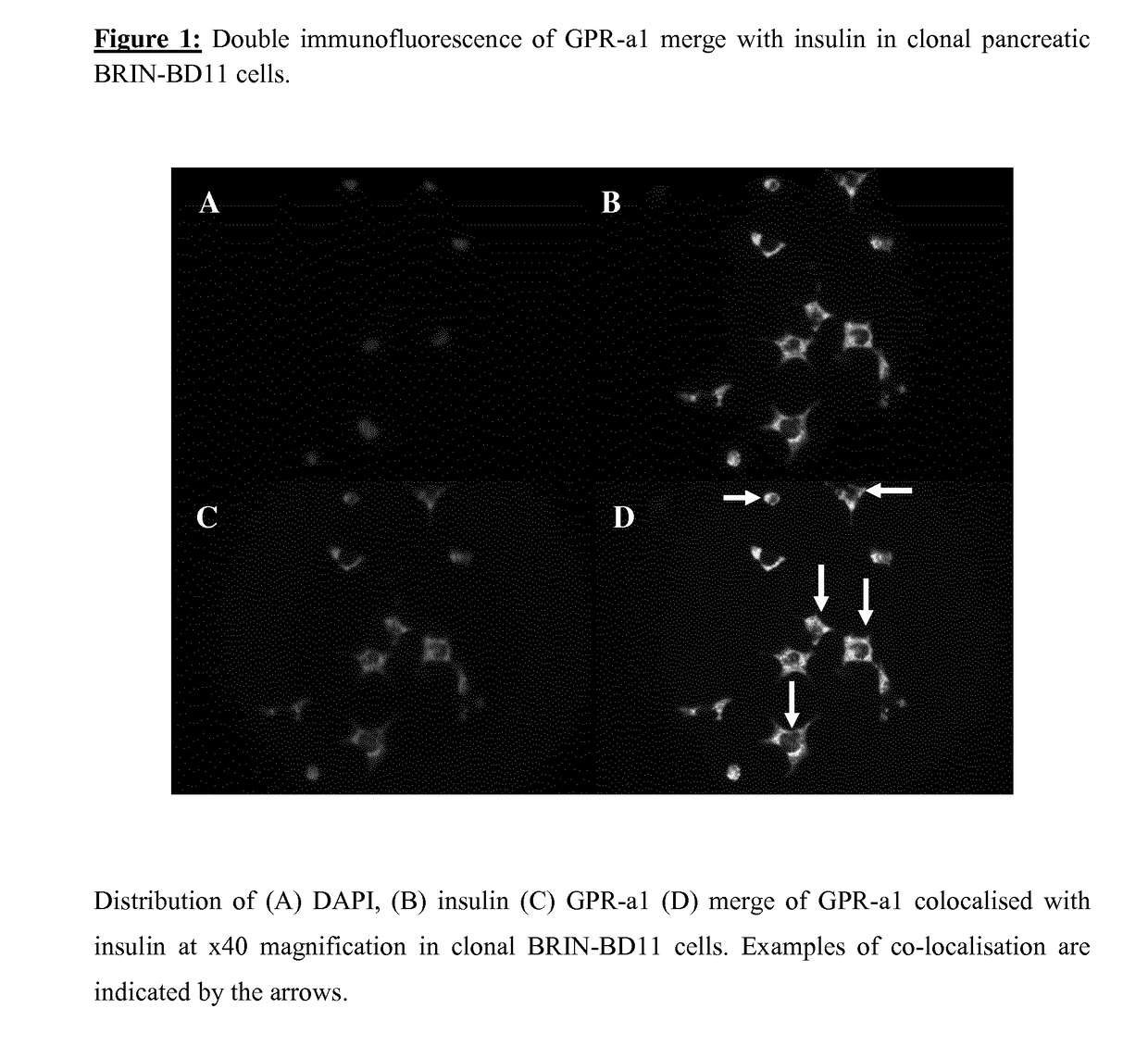
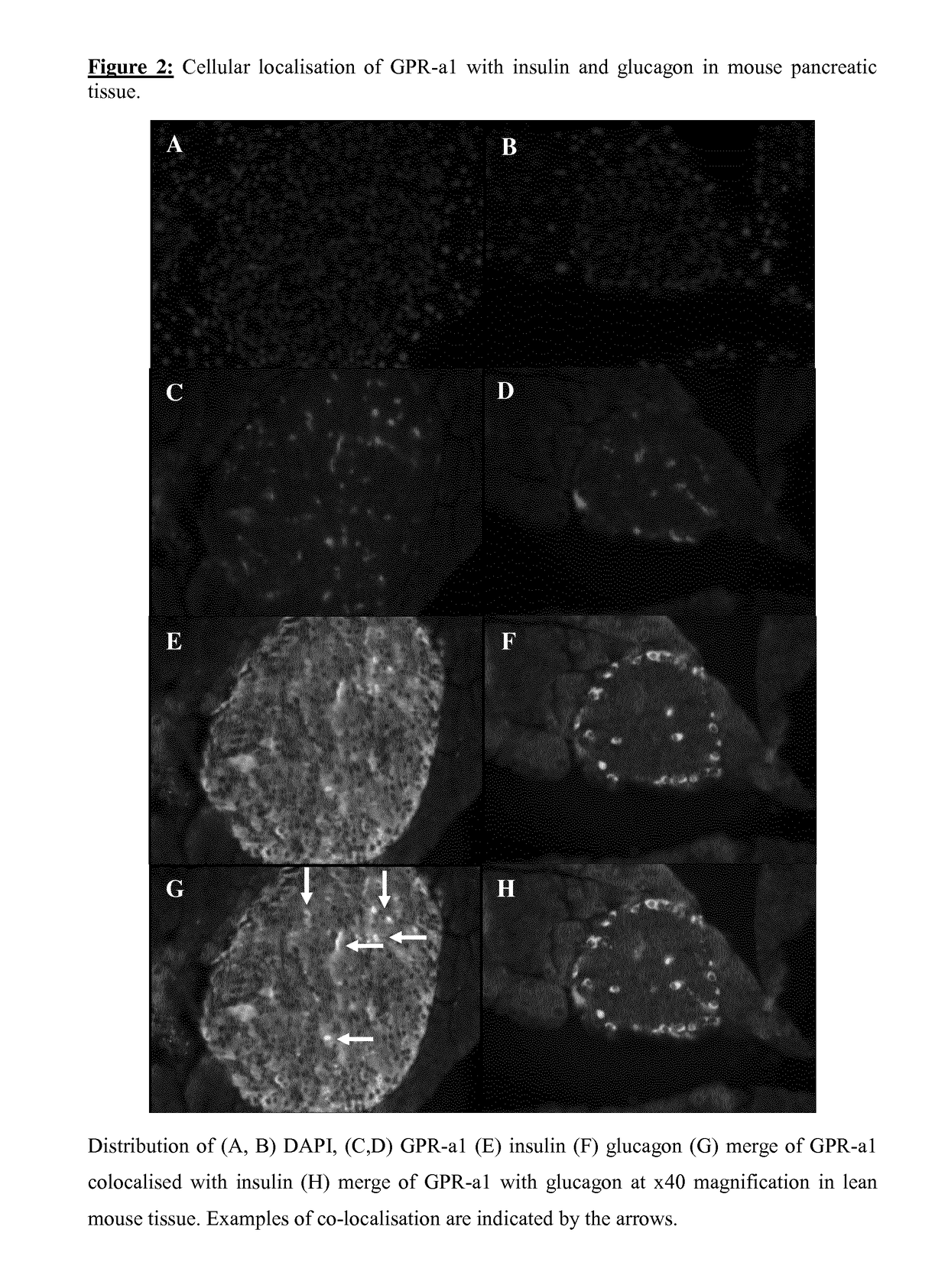
[0105]Distribution of insulin and GPR84 were investigated in BRIN-BD11 cells. DAPI (blue) stained nuclei (FIG. 1A), and insulin (green) were distributed across the BRIN-BD11 cells (FIG. 1B) with a similar staining pattern to GPR84 (red) (FIG. 1C). Double immunofluorescence combination of insulin with GPR84 indicated areas of co-localisation (yellow) (FIG. 1D), demonstrating the presence of GPR-a1 in pancreatic beta cells. The distribution of DAPI, insulin, glucagon and GPR84 in mouse pancreatic islets are shown in FIG. 2. DAPI (blue) displayed the nuclei in pancreatic islets (FIGS. 2A, B), GPR84 (green) was expressed throughout the islet with a similar staining pattern to insulin (red) (FIGS. 2C, D). Merge of insulin and GPR84 indicated that insulin secreting beta cells express GPR84 (FIG. 2G) while there was no evidence of the GPR84 receptor in glucagon secreting alpha cells (FIG. 2H). While no co-localisation was displayed on ...
example 2
Effects of Compositions of the Present Invention on Insulin Secretion from BRIN-BD11 Cells
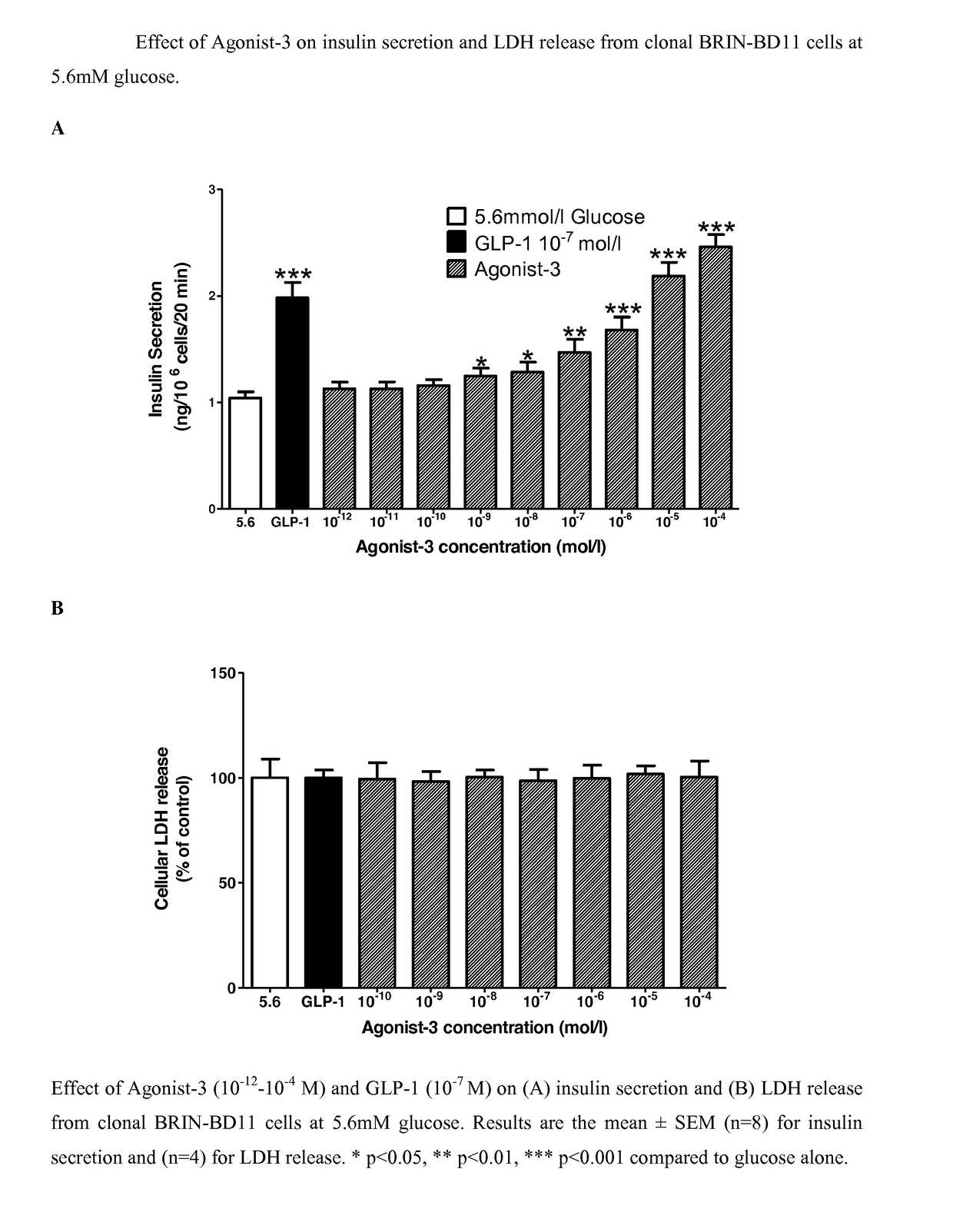
[0106]Insulin releasing properties of compositions of the present invention at 10−12-10−4 mol / L were assessed in clonal BRIN-BD11 cells at 5.6 mM and 16.7 mM glucose. Diindolylmethane at 10−8-10−4 mol / L enhanced insulin release (EC50 1.3×10−7 mol / L) (p−9-10−4 mol / L (EC50 1.0×10−6 mol / L) (p<0.05-p<0.001) (FIG. 4A). No cytotoxicity was found with Diindolylmethane at 5.6 mM glucose (FIG. 3B) and 16.7 mM glucose (FIG. 4B).
[0107]Embelin at 10−9-10−4 mol / L enhanced insulin release (p−7 mol / L) (FIG. 5A) at 5.6 mM basal glucose concentrations. At stimulatory glucose concentrations (16.7 mM glucose), Embelin enhanced insulin release at 10−10-10−4 mol / L (p−7 mol / L) (FIG. 6A). No cytotoxicity was found with Embelin at 5.6 mM glucose (FIG. 5B) and 16.7 mM glucose (FIG. 6B).
[0108]Indole-3-carbinol at 10−7-10−4 mol / L enhanced insulin release (p−6 mol / L) at 5.6 mM basal glucose concentrations. At stimulatory ...
example 3
Effect of Compositions of the Present Invention on Intracellular Ca2+ and cAMP in BRIN-BD11 Cells
[0112]For confirmation of the stimulatory ability of compositions of the present invention on insulin secretion in pancreatic islets and to examine the mechanism of action, beta stimulus coupling pathways and changes in intracellular calcium concentrations and cAMP production in pancreatic BRIN-BD11 cells were examined.
[0113]At both basal and stimulatory glucose concentrations, compositions of the present invention (10−4 mol / L) augmented intracellular Ca2+ concentrations at 5.6 mM glucose (p<0.05-p<0.001) (FIGS. 7-10) with the exception of Indole-3-carbinol and [6]-gingerol. At 16.7 mM glucose, Diindolylmethane, Embelin and [6]-shogaol increased intracellular Ca2+ concentrations (p<0.001) (FIG. 10).
[0114]As shown in FIG. 11, the stimulatory action of Diindolylmethane and Embelin on the insulin secretory pathway involves the cAMP-dependent pathway in pancreatic islets. [6]-shogaol and med...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molar density | aaaaa | aaaaa |
| Molar density | aaaaa | aaaaa |
| Molality | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap