Anti-pad2 antibody for treating and evaluating rheumatoid arthritis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
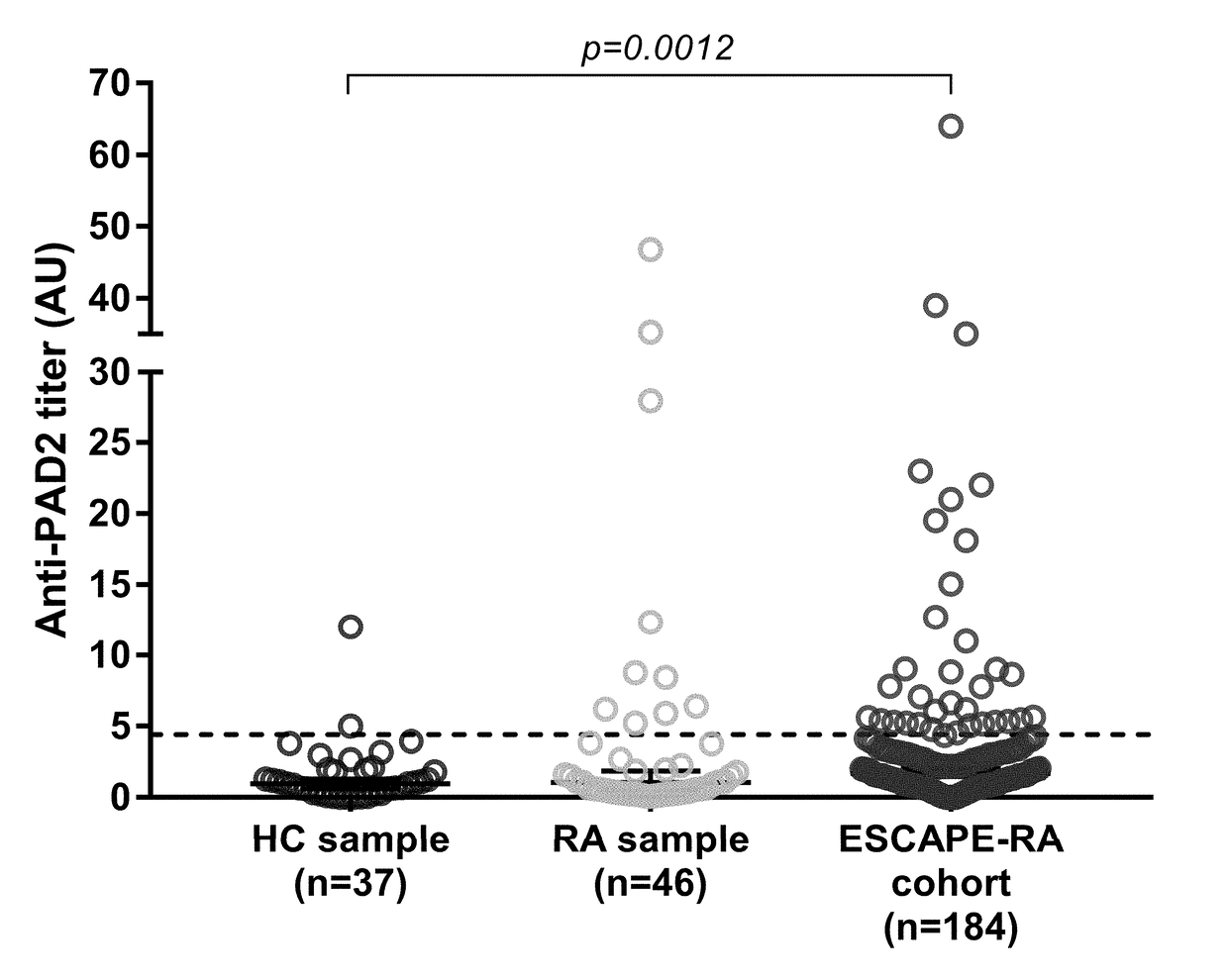
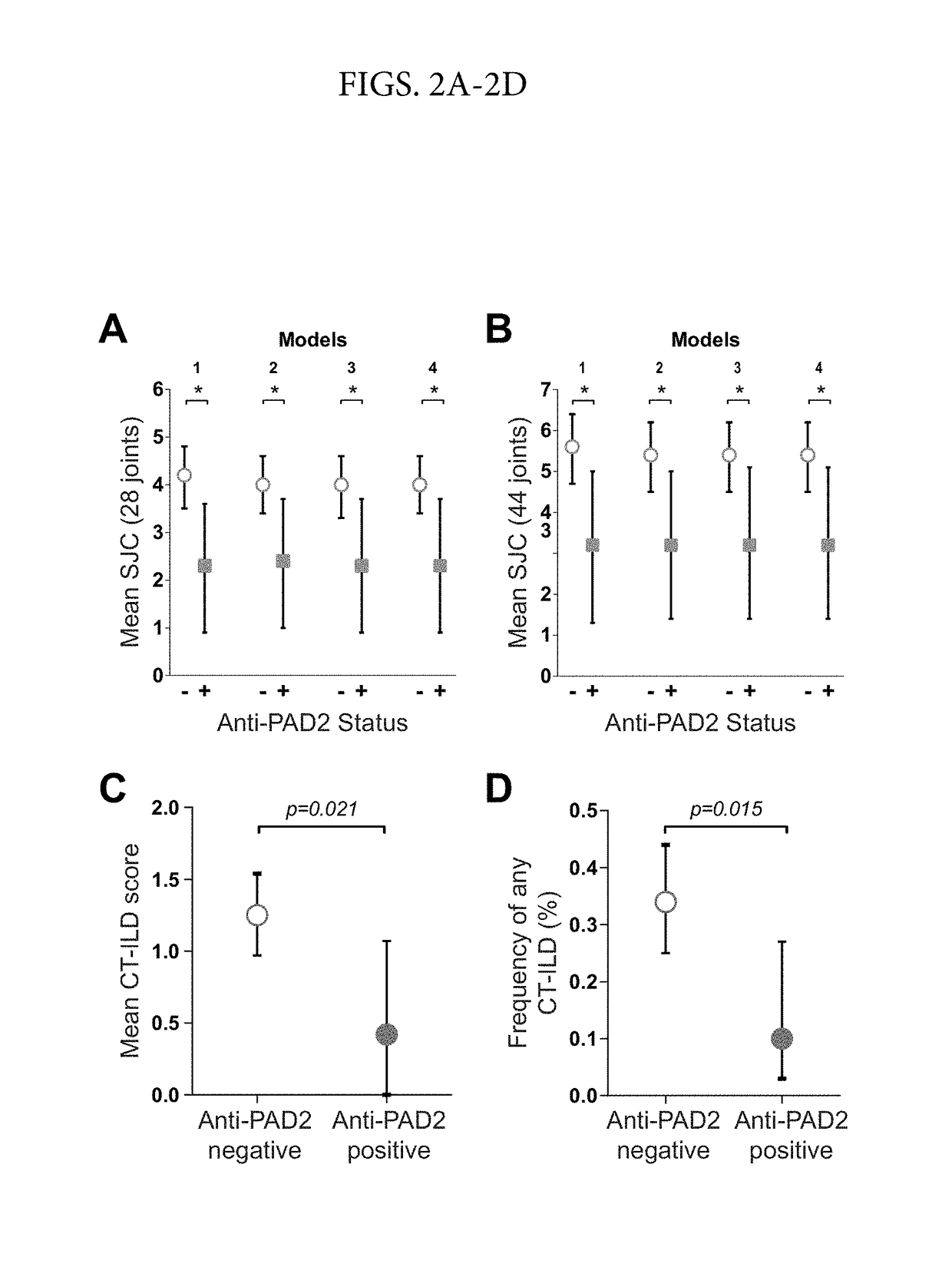
[0070]Autoantibodies to Peptidylarginine Deiminase 2 are Associated with Less Severe Disease in Rheumatoid Arthritis
[0071]In this study, we found that anti-PAD2 antibodies are present in a subset of patients with RA who lack the traditional risk factors associated with severe disease, such as ACPAs and SE. Instead, they have a unique set of demographic and clinical characteristics including less progressive articular damage and lower risk of ILD. These findings have important implications in the ability to identify clinically informative patient subgroups to aid in disease prognosis and selection of appropriate therapeutic agents.
Methods
[0072]Human Subjects
[0073]Convenience sera from 37 healthy controls and 42 patients with RA from the Johns Hopkins Arthritis Center were used as a discovery sample for anti-PAD2 antibodies. Sera from 184 RA patients from the Evaluation of Subclinical Cardiovascular Disease and Predictors of Events in Rheumatoid Arthritis (ESCAPE RA) cohort were then ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com