Syd985 treatment of t-dm1 refractory cancer patients
a technology of t-dm1 and refractory cancer, which is applied in the field of t-dm1 refractory breast cancer patients, can solve the problems of tumor cell death, severe problems of heart, liver and lung, and nucleic acid architectur
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
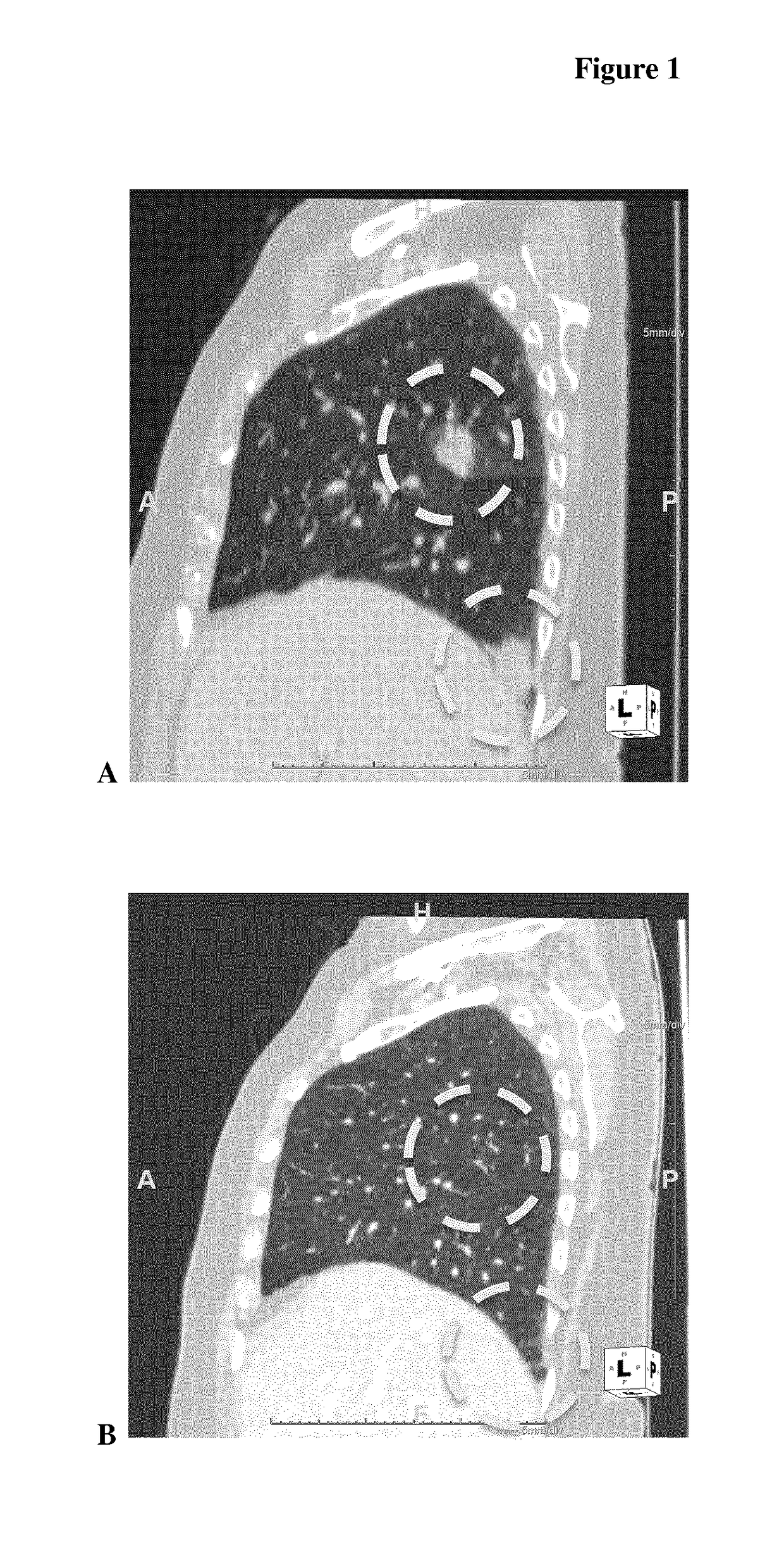
[0060]First-in-Human Clinical Study
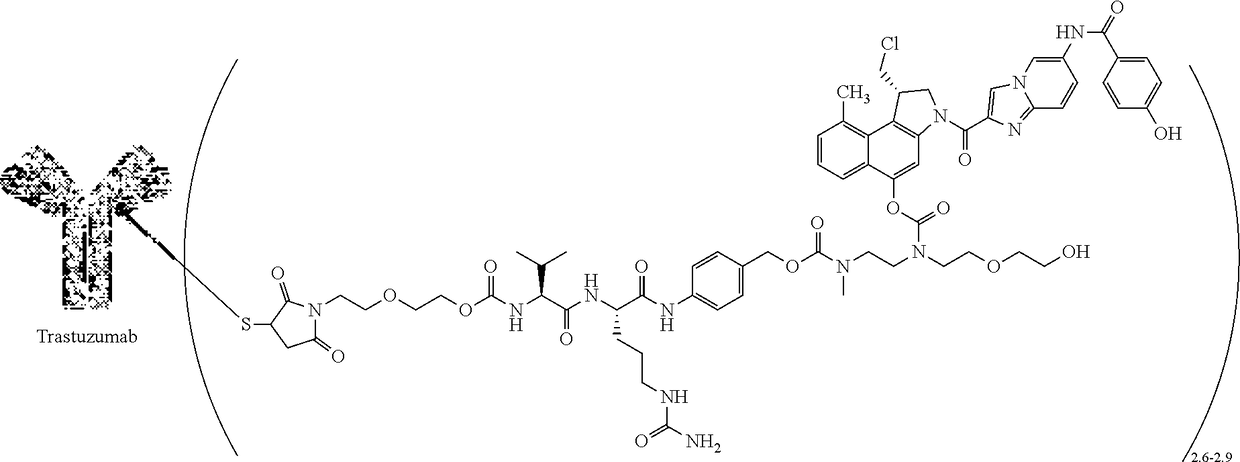
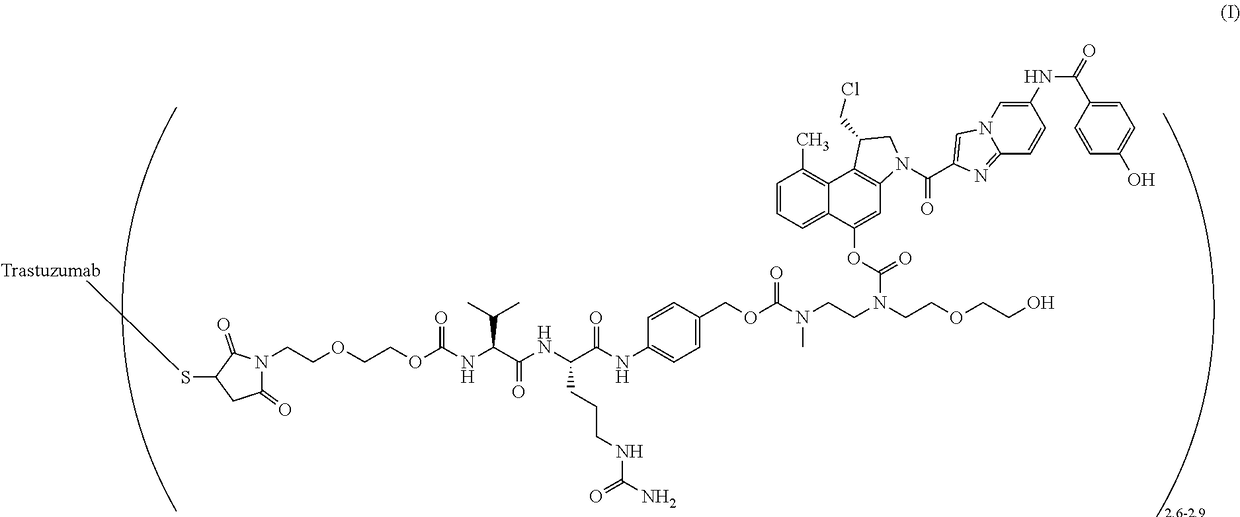
[0061]A two-part first-in-human phase I study (with expanded cohorts) with the antibody-drug conjugate SYD985 (trastuzumab vc-seco-DUBA), having an average DAR of about 2.8, was started to evaluate the safety, pharmacokinetics and efficacy in patients with locally advanced or metastatic solid tumors (i.e., NCT02277717). Part I is the dose-escalation part in which a low dose of SYD985 was given to three or six cancer patients (females or males having solid tumors of any origin). If it were well tolerated, a higher dose of SYD985 was given to three or six other cancer patients. This continued until it was not safe anymore to increase the dose further. Any dose level could be extended with additional patients. In part II of the study, several groups of patients with a specific type of cancer (including breast and gastric tumors) will receive the SYD985 dose selected for further development. All patients from both parts of the study will receive SYD985...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| refractory | aaaaa | aaaaa |
| body weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com